
Applying stem cell therapy in intractable diseases: a narrative review of decades of progress and challenges
Introduction
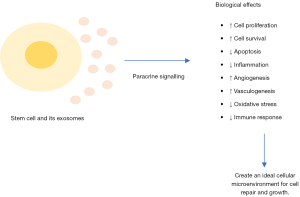
With the rapid advancement and development in regenerative medicine, the ability to replace or repair faulty cells, tissues and even organs is steadily becoming a tangible reality. The ever-evolving field of regenerative medicine aims to treat and cure diseases by using living cells to either replace or repair diseased and damaged areas of medical interest. Among the many branches of regenerative medicine such as tissue engineering and organ regeneration, stem cell therapy (SCT) is regarded as the frontier of regenerative medicine. SCT is a therapeutic tool used to treat or prevent diseases through the use of human stem cells such as embryonic stem cells (ESCs), induced pluripotent stem cells (iPSCs) and adult stem cells (ASCs). These stem cells have the potential to heal or regenerate damaged body tissues and congenital defects due to their infinite ability to self-divide into various types of cells. Stem cells also act indirectly in tissue repair and regeneration by releasing paracrine factors stored in exosomes such as cytokines, chemokines, growth factors and extracellular matrix molecules catalysing the repair of injured tissues, as shown in Figure 1 (1,2). Hence, in certain cases, exosomes derived from stem cells (largely mesenchymal stem cells, MSCs) would be administered to patients instead of purified stem cells. Exosomes are extracellular vesicles released from stem cells that play a role in the mediation of cell-to-cell signalling through the release of paracrine factors, catalysing the repair and regeneration of neighbouring cells (3). Exosomes are also able to penetrate the blood-brain barrier and blood-spinal cord barrier with high transport efficiency and most importantly, without occluding microvessels as they are small-sized molecules (4). This cell-free therapy allows patients to benefit from the reparative effects of stem cells, without introducing living stem cells that can be potentially rejected by the body and have tumourigenic properties. Nevertheless, the techniques of isolating and purifying exosomes are yet to be perfected (5).
The world’s first documented SCT was haematopoietic stem cell transplantation (HSCT) involving grafted bone marrow for leukaemia, reported in 1956 (6). Since then, many milestones have been achieved in the field of SCT (Figure 2). SCT has been applied in various intractable diseases such as haematological diseases (17,18), neurological diseases (19,20), diabetes mellitus (21,22), retinal degenerative disorders (23,24) and COVID-19 infections (25,26). With positive therapeutic outcomes of stem cell research, various stem cell techniques have been innovated such as stem cell isolation methods and in vitro cell culture protocols (7,9). A breakthrough in stem cell research was achieved when human ESCs were successfully cultivated through in vitro culture (9). However, as ESCs are obtained from the inner cell mass of destroyed blastocysts, many activists, ranging from politicians, religious leaders, and human rights advocates, opposed the use of human embryos—halting the progression of stem cell research (27). Fortunately, this ethical controversy was circumvented by reprogramming fibroblasts into precursor ESCs by using specific genes Oct3/4, Sox2, c-Myc and Klf4 (10). These reprogrammed cells, known as iPSCs, share similar morphology, growth properties and cell marker genes as ESCs. Besides obsoleting the need for embryos, another advantage of using iPSCs in SCT as opposed to using ESCs is that there will be a decreased risk of immune rejection following transplantation as iPSCs are generated directly from the patient’s somatic cells. The earliest iPSC therapy was performed on patients suffering from age-related macular degeneration (12). Presently, state of the art technology such as CRISPR-Cas 9 (clustered regularly interspaced short palindromic repeats- associated protein 9) gene-editing tool is utilised in SCT research due to its finer gene editing and easy design (28,29).
Stem cell-based therapies are regulated independently by various regulatory bodies in different countries (30). Thus, the regulatory and legal frameworks governing the use of SCT differ from country to country. Nonetheless, all the frameworks are in favour of SCT research and commercialisation while monitoring the safety and ethical issues of stem cell-based therapies. As of 2022, the only established therapy that is recognised by the Food and Drug Administration (FDA) for use in the United States is HSCT to treat blood and immune disorders (31). Among the stem cell-based therapies approved in other countries are Holoclar® by the European Union for eye burns (32), Stempeucel® by India for Buerger’s disease (a type of obstructive vascular disease) (33), Neuronata-R® by South Korea for amyotrophic lateral sclerosis (ALS) (13), Stemirac® by Japan for spinal cord injuries (SCI) (14) and Prochymal by Canada to treat Graft versus Host Disease (GvHD) (34). However, despite showing promising results, many SCTs are yet to be approved by regulatory bodies due to various concerns. While there are articles outlining the current updates of SCT (35-40), many of them focuses on one disease or provides a brief account on the application of SCT in several diseases. Therein, this review aims to provide a comprehensive outlook covering the progress of SCT in treating various diseases with high morbidity and mortality as well as the main challenges faced by stem cell researchers such as uncertainty in the underlying stem cell mechanism, ethical issues, genetic instability and immune rejection. We present the following article in accordance with the Narrative Review reporting checklist (available at https://sci.amegroups.com/article/view/10.21037/sci-2022-021/rc).
Methods
An extensive search was performed using Google scholar, PubMed and clinical studies database (https://clinicaltrials.gov/). The latest literatures search was done from June 2021 until March 2022 using the following keywords: ‘stem cell therapy’, ‘MSC’, ‘ESC’, ‘iPSC’, ‘stem cells’, ‘exosomes’, ‘clinical challenges’ along with the interested disease name. All studies ranging from in vitro studies, in vivo studies, case reports and clinical trial studies were used in this review, provided that they were published in English and had undergone peer review.
The origin, sources and types of therapeutic stem cells
The first development of stem cells begins after the formation of a blastocyst, following the fusion of an oocyte and a sperm (36). A blastocyte consists of two cell types; the inner cell mass containing ESCs which support foetal development, and the trophectoderm which is necessary for placenta formation. ESCs, which are pluripotent in nature, are able to differentiate into the three germ layers (ectoderm, endoderm and mesoderm) that give rise to various differentiated foetal cells and tissues in a process known as gastrulation. ESCs will disappear after the formation of these three germ layers (41). During gastrulation, the ectoderm layer differentiates into nervous system tissues and the epidermis, the endoderm gives rise to the digestive and respiratory systems while the mesoderm develops into skeletal, muscular and connective tissues to form the rest of the tissues such as the heart, bones and urogenital tissues, forming a fully developed foetus. Following the formation of tissues, multipotent ASCs can be found in specific tissues. ASCs are able to differentiate into multiple specialised cell types based on their tissue niches (37,42). ASCs are broadly divided into two categories, haematopoietic stem cells (HSCs) and MSCs (43). Other sources of ASCs included stem cells derived from the brain, adipose tissues and the skin. Undifferentiated ASCs will either remain dormant or begin to self-renew and differentiate into committed cells specific to their host tissues, depending on the stimuli received (42). Types of stimuli include ischemia, cellular damage, inflammation and cytokine signalling. As ASCs are scarcely found in the body, in vitro stem cell expansion is crucial for therapeutic purposes.
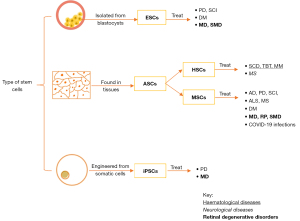
Therapeutic stem cells are sourced from various parts of the human body depending on the intended application. Historically, stem cells are broadly divided into two categories: ESCs and ASCs (44). However, with the discovery of iPSCs, it is now known that stem cells can be engineered from somatic cells (10). The characteristics and differences between ESCs, ASCs and iPSCs are outlined in Table 1. The types of stem cells used in SCT are carefully chosen by researchers by weighing their advantages and disadvantages. Hence, different types of stem cells are used in treating specific diseases as illustrated in Figure 3.
Table 1
| Characteristics | ESCs | ASCs | iPSCs |
|---|---|---|---|
| Source | Inner cell mass of blastocyst (27) | Niches in body tissues (41) | Adult somatic cells (10) |
| Potency | Pluripotent (27) | Multipotent (42) | Pluripotent (10) |
| Able to differentiate into all three germ layers; ectoderm, endoderm and mesoderm | Able to differentiate into multiple specialised cell types based on tissue niche such as MSCs, HSCs, NSCs | Able to differentiate into all three germ layers; ectoderm, endoderm and mesoderm | |
| Able to differentiate into all types of cells | Able to differentiate into all types of cells | ||
| Risk of immune rejection | High (38) | Low (38) | Low (38) |
| Risk of teratoma formation | High (38) | Low (38) | High (45) |
| Ethical issues | Present due to the use of embryos and potential use for human cloning (45) | Absent (38) | Present due to the potential use for human cloning (46) |
ESCs, embryonic stem cells; ASCs, adult stem cells; iPSCs, induced pluripotent stem cells; MSCs, mesenchymal stem cells; HSCs, haematopoietic stem cells; NSCs, neural stem cells.
Current updates in stem cell therapy in various diseases
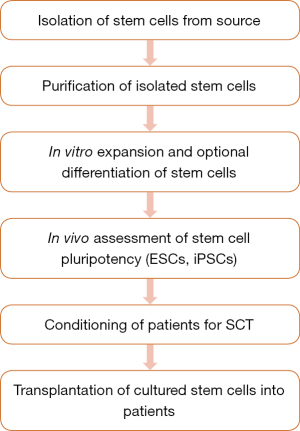
In hopes of finding a cure, the potential of ESCs, ASCs and iPSCs in treating various diseases is heavily researched. SCT is especially researched as a promising curative treatment for intractable diseases with heavy disease burden such as haematological diseases, neurological diseases, diabetes mellitus, retinal degenerative disorders and COVID-19 infections. Hence, the current developments and applications of SCT in treating these intractable diseases are catalogued in the following sections while the fundamental steps involved in therapeutic stem cell preparation for patients undergoing SCT is outlined in Figure 4 (47).
Haematological diseases
Haematological diseases are conditions affecting the components of the blood and eventually the circulatory system. Currently, the curative therapy for haematological diseases is autologous or allogeneic HSCT with the aim to reconstitute and replace defective blood components (17). HSCT is the most widely used and established SCT in treating blood disorders. Nonetheless, there are a few challenges in using HSCT as a treatment option. For instance, adult patients undergoing HSCT with HSCs derived from umbilical cords will require multiple units of blood due to the insufficient number of stem cells in one unit (48). In 2014, Sauvageau and his team (48) identified a molecule, UM 171, capable of replicating the number of stem cells. Their ground-breaking finding increases the availability of cord blood stem cells for transplantation while decreasing the risk of post-transplantation complications as only one cord blood source is needed. The safety and feasibility of the use of UM171 in the expansion of cord blood stem cells have been validated by clinical trials (18,49). Subsequent studies revealed that UM171 restores epigenetic marks, H3K4me2 and H3K27ac, which are reduced following ex vivo cultivation (50). Likewise, substantial research is undertaken to circumvent the limitation of existing SCT and discover novel SCT to treat haematological diseases such as sickle cell disease (SCD), transfusion-dependent beta-thalassemia (TBT) and multiple myeloma (MM). Ongoing clinical trials evaluating the potential of SCT in treating these three diseases are tabulated in Table 2.
Table 2
| Disease | Type of stem cells | Clinical trial identifier | Title of clinical trial | Method of treatment | Commencement of clinical trial | Status of clinical trial |
|---|---|---|---|---|---|---|
| SCD | HSCs | NCT03653247 | A Study to Assess the Safety, Tolerability, and Efficacy of BIVV003 for Autologous Hematopoietic Stem Cell Transplantation in Patients With Severe Sickle Cell Disease | Subcutaneous injection of autologous gene-edited (CD)34+ hematopoietic stem cells. Product known as BIVV003 | 2019 | Recruiting |
| HSCs + progenitor cells | NCT04443907 | Study of Safety and Efficacy of Genome-edited Hematopoietic Stem and Progenitor Cells in Sickle Cell Disease (SCD) | Intravenous infusion of autologous gene-edited (CD)34+ hematopoietic stem cells. Product known as OTQ923 or HIX763 | 2020 | Recruiting | |
| HSCs | NCT04853576 | EDIT-301 for Autologous HSCT in Subjects With Severe Sickle Cell Disease | Intravenous infusion of autologous gene-edited (CD)34+ hematopoietic stem cells. Product known as EDIT-301 | 2021 | Recruiting | |
| HSCs | NCT04819841 | Gene Correction in Autologous CD34+ Hematopoietic Stem Cells (HbS to HbA) to Treat Severe Sickle Cell Disease (CEDAR) | Intravenous infusion of autologous gene-edited (CD)34+ hematopoietic stem cells. Product known as GPH101 | 2021 | Recruiting | |
| TBT | HSCs | NCT03207009 | A Study Evaluating the Efficacy and Safety of the LentiGlobin® BB305 Drug Product in Participants With Transfusion-Dependent β-Thalassemia | Intravenous infusion of autologous gene-edited (CD)34+ hematopoietic stem cells/progenitor cells. Product known as LentiGlobin BB305 | 2017 | Active, not recruiting |
| HSCs/progenitor cells | NCT03432364 | A Study to Assess the Safety, Tolerability, and Efficacy of ST-400 for Treatment of Transfusion-Dependent Beta-thalassemia (TDT) | Intravenous infusion of autologous gene-edited (CD)34+ hematopoietic stem cells/progenitor cells. Product known as ST-400 | 2018 | Active, not recruiting | |
| HSCs | NCT05444894 | EDIT-301 for Autologous Hematopoietic Stem Cell Transplant (HSCT) in Participants With Transfusion-Dependent Beta Thalassemia (TDT) | Intravenous infusion of autologous gene-edited (CD)34+ hematopoietic stem cells/progenitor cells. Product known as EDIT-301 | 2022 | Recruiting | |
| HSCs | NCT05477563 | Evaluation of Efficacy and Safety of a Single Dose of CTX001 in Participants With Transfusion-Dependent β-Thalassemia and Severe Sickle Cell Disease | Intravenous infusion of autologous gene-edited (CD)34+ hematopoietic stem cells/progenitor cells. Product known as CTX001 | 2022 | Not yet recruiting | |
| MM | HSCs | NCT03292263 | ASCT With Nivolumab in Patients With Multiple Myeloma | Intravenous infusion of peripheral stem cells along with PD-1 inhibitor (Nivolumab) | 2017 | Recruiting |
| HSCs | NCT03127761 | Assessment of Allogeneic Hematopoietic Cell Transplantation in Medicare Beneficiaries With Multiple Myeloma | Transplantation of allogeneic HSC | 2017 | Recruiting |
SCD, sickle cell disease; HSCs, haematopoietic stem cells; TBT, transfusion-dependent beta-thalassemia; MM, multiple myeloma; PD-1, programmed death-1.
SCD and TBT are life-threatening genetic disorders caused by a single mutation affecting oxygen transport in the body. The curative treatment of SCD and TBT is allogeneic HSCT which is dependent on donor availability and possesses a high risk of GvHD (51,52). It was found that maintaining a high level of foetal haemoglobin will ameliorate SCD and TBT (53). Therefore, the potential of transplanting gene-edited haematopoietic stem and progenitor cells (HSPCs) as a treatment option was investigated in two patients with SCD and TBT respectively (54). As SCD and TBT are monogenic mutations, gene editing was feasible. CRISPR-Cas 9 gene editing was used to express BCL11A genes in HSPCs, increasing the expression of foetal haemoglobin. While there were a few resolved adverse reactions post-transplantation, both patients had their last blood transfusion a few weeks after the procedure and had normal blood levels during follow-ups. Thus, results indicate that the gene edited HSPC transplantation was safe and efficacious in the short run. Another study employed CRISPR-Cas 9 to perform gene correction of β-globin gene in autologous HSPCs in their attempt to treat SCD patients (55). It was reported that 60% of allelic correction was achieved during manufacturing and after transplanting the corrected HSPCs into mice, 20% gene correction was attained with negative tumorigenicity.
MM is a malignancy of plasma B cells (56). Due to the abnormal proliferation of plasma cells, MM patients experience fatigue, osteomalacia and kidney failure. Most MM patients undergo autologous or allogeneic stem cell transplantation in the hopes of curing the disease but oftentimes, they experience relapses (57). Based on ESMO guidelines, autologous stem cell transplantation (ASCT) should be performed on patients less than 70 years old (58). Herein, a retrospective study was carried out to evaluate the efficacy of ASCT in patients above 75 years old receiving melphalan (59). The study included 50 participants with one participant dying within 100 days after transplantation due to arrhythmia. It was concluded that ASCT is safe to be performed in the said group, with careful evaluation. Past research demonstrated cytotoxic effects of natural killer (NK) cells against MM cells, especially those derived from allogeneic sources (60). However, a meta-analysis study demonstrated that allogeneic stem cell transplantation is not favourable in newly diagnosed and relapsed MM patients but is advantageous in high-risk MM patients with low long-term survival rates (61). Therefore, Shah et al. (62) designed a clinical study to evaluate the feasibility and safety of cord blood-derived NK cells infusion along with autologous stem cell engraftment in treating 12 MM patients. The NK cells engraftment was accepted and well tolerated by all patients with ten patients showed positive outcomes. However, after 21 months, four patients have either progressed or relapsed. Hence, the efficacy of the treatment is indeterminable.
Neurological diseases
The increasing prevalence of neurological diseases, especially among the elderly population is alarming governments and communities worldwide (62). Based on the Global Burden of Disease Study in 2016, neurological diseases have the highest morbidity and disability-adjusted life year (DALY). To alleviate this worrying problem, many researchers are looking into SCT as a potential treatment for neurological diseases. Studies show that SCT will not only slow down the progression of neurodegenerative diseases such as Alzheimer’s disease (AD), Parkinson’s disease (PD), Huntington’s disease (HD), spinal cord injury (SCI), ALS and multiple sclerosis (MS) but it has the potential to reverse the underlying disease pathogenesis. Currently, several clinical trials are being carried out to ascertain the therapeutic effects of SCT (mostly using MSCs) in treating these neurological diseases as shown in Table 3.
Table 3
| Disease | Type of stem cells | Clinical trial identifier | Title of clinical trial | Method of treatment | Commencement of clinical trial | Status of clinical trial |
|---|---|---|---|---|---|---|
| AD | MSCs | NCT02833792 | Allogeneic Human Mesenchymal Stem Cells for Alzheimer’s Disease | Intravenous infusion of allogeneic human MSCs | 2016 | Recruiting |
| MSCs | NCT03117738 | A Study to Evaluate the Safety and Efficacy of AstroStem in Treatment of Alzheimer’s Disease | Intravenous infusion of autologous adipose tissue derived MSCs. Product known as Astrostem | 2017 | Completed | |
| MSCs | NCT04040348 | Alzheimer’s Disease Stem Cells Multiple Infusions | Intravenous infusion of UC-MSCs | 2019 | Active, not recruiting | |
| PD | MSCs | NCT02611167 | Allogeneic Bone Marrow-Derived Mesenchymal Stem Cell Therapy for Idiopathic Parkinson’s Disease | Intravenous infusion of allogeneic BM-MSCs | 2015 | Completed |
| MSCs | NCT03550183 | Umbilical Cord Derived Mesenchymal Stem Cells Therapy in Parkinson’s Disease | Intravenous infusion of UC-MSCs | 2018 | Enrolling by invitation | |
| MSCs | NCT04506073 | Phase IIa Randomized Placebo Controlled Trial: Mesenchymal Stem Cells as a Disease-modifying Therapy for iPD | Intravenous infusion of allogeneic BM-MSCs | 2020 | Active, not recruiting | |
| MSCs | NCT05152394 | Safety of Cultured Allogeneic Adult Umbilical Cord Derived Mesenchymal Stem Cells for Parkinson’s Disease | Intravenous infusion of allogeneic adult UC-MSCs | 2021 | Not yet recruiting | |
| MSCs | NCT04928287 | Randomized, Double-Blind Clinical Trial for Parkinson’s Disease (Early and Moderate) (PD) | Intravenous infusion of autologous adipose-derived MSCs | 2021 | Active, not recruiting | |
| SCI | MSCs | NCT03003364 | Intrathecal Administration of Expanded Wharton’s Jelly Mesenchymal Stem Cells in Chronic Traumatic Spinal Cord Injury | Intrathecal infusion of Wharton’s jelly-derived MSCs | 2016 | Completed |
| MSCs | NCT03308565 | Adipose Stem Cells for Traumatic Spinal Cord Injury (CELLTOP) | Intrathecal infusion of autologous adipose-derived MSCs | 2017 | Active, not recruiting | |
| MSCs | NCT05152290 | Safety of Cultured Allogeneic Adult Umbilical Cord Derived Mesenchymal Stem Cells for SCI | Intravenous and intrathecal infusion of allogeneic adult UC-MSCs | 2021 | Recruiting | |
| ALS | MSCs | NCT02383654 | Compassionate Treatment: An Exploratory Clinical Trial to Assess Treatment of Amyotrophic Lateral Sclerosis | Intracerebral and intravenous infusion of autologous adipose-derived MSCs | 2015 | Completed |
| MSCs | NCT03828123 | Autologous Multipotent Mesenchymal Stromal Cells in the Treatment of Amyotrophic Lateral Sclerosis (AMSC-ALS-001) | Intrathecal infusion of autologous adipose-derived MSCs | 2019 | Completed | |
| MSCs | NCT04651855 | The Evaluation of the Effect of Mesenchymal Stem Cells on the Immune System of Patients With ALS (ALSTEM) | Intrathecal infusion of Wharton’s jelly-derived MSCs | 2020 | Active, not recruiting | |
| MSCs | NCT04745299 | Evaluation the Efficacy and Safety of Multiple Lenzumestrocel (Neuronata-R® Inj.) Treatment in Patients With ALS (ALSummit) | Intrathecal infusion of autologous BM-MSCs. Product known as Lenzumestrocel | 2021 | Recruiting | |
| MSCs | NCT04821479 | Repeated Mesenchymal Stem Cell Injections in ALS | Intrathecal infusion of autologous BM-MSCs | 2021 | Completed | |
| MS | MSCs | NCT03326505 | Allogenic Mesenchymal Stem Cells And Physical Therapy for MS Treatment | Intrathecal infusion of allogenic UC-MSCs | 2017 | Completed |
| NSCs | NCT03269071 | Neural Stem Cell Transplantation in Multiple Sclerosis Patients (STEMS) | Intrathecal infusion of human fetal-derived NSCs | 2017 | Completed | |
| MSCs | NCT05003388 | Safety of Cultured Allogeneic Adult Umbilical Cord Derived Mesenchymal Stem Cell Intravenous Infusion for MS | Intravenous infusion of allogeneic adult UC-MSCs | 2021 | Recruiting | |
| MSCs | NCT04749667 | Study of Mesenchymal Autologous Stem Cells as Regenerative Treatment for Multiple Sclerosis (SMART-MS) | Intrathecal infusion of autologous BM-MSCs | 2021 | Recruiting |
AD, Alzheimer’s disease; MSC, mesenchymal stem cell; PD, Parkinson’s disease; SCI, spinal cord injury; ALS, amyotrophic lateral sclerosis; MS, multiple sclerosis; UC-MSC, umbilical cord-derived MSC.
Alzheimer’s disease (AD)
AD is a debilitating condition affecting one’s memory and cognitive function. AD, a progressive neurodegenerative disease, is characterised by accumulation of beta-amyloid plaques that induces neuronal loss and death (19,63). Hence, patients with AD experience a rapid decline in cognitive impairment leading them to be highly dependent on others for basic needs. Until now, only one FDA-approved drug has the capability to reverse AD through the removal of amyloid plaques (64), while the rest of the drugs available can only slow down cognitive impairment and neuronal loss (65). The advancement in SCT provides hope for AD patients to lead a disease-free life. In vitro studies have shown that transplanted stem cells release protective factors and regulate neuroinflammatory response via paracrine actions, aiding in the reversal of neuropathological alteration of AD (63-70).
The first clinical trial involving the use of MSCs to treat AD was documented in 2015 (71). The study involving nine AD patients concluded that MSCs derived from human umbilical cord blood is safe to be used to treat AD as it does not cause adverse effects to patients. However, there were no positive changes in the said nine patients. Nevertheless, their findings paved the way for future clinical trials involving MSCs in AD treatment (ClinicalTrial.gov Identifier: NCT02600130, NCTO4855955, NCT04388982). In 2020, animal studies revealed that weekly doses of cultivated MSCs exposed to AD brain homogenate through the intranasal route brought positive clinical outcomes in mice (66). Complete restoration of mice memory was reported following the treatment and post-mortem examination revealed a decrease in neuroinflammation (evaluated using immunostaining), plaque load and aggregation. Similar results were achieved by other studies (63,70).
In another study, marked improvement in the brain glucose metabolism and cognitive function in transgenic mice was observed following administration of MSC-derived exosomes (69). Moreover, it was found that MSC-derived exosomes promoted the regulation of neurons and astrocyte phases in the brain. SCT involving neural stem cells (NSCs) is another potential AD treatment. McGinley et al. (67) found that NSCs improved cognitive ability and decreased plaques in mice as long as after six weeks post-transplantation via the fimbria fornix in the hippocampus. Similar research involving human neural stem and progenitor cells (NPSCs) revealed intracerebral transplantation of NPSCs took a longer time to work compared to via systemic transplantation, but in both procedures, mice had marked improvement in memory and increased neuronal density (68). Hence, the effects of SCT in treating AD are highly debatable as it depends on the disease stage, cell isolation and culturing techniques, source of stem cells and model of neurodegeneration.
Parkinson’s disease (PD)
PD is a progressive neurodegenerative disease affecting motor control and cognitive abilities, caused by the rapid loss of neurons in the substantia nigra responsible for dopamine production (72,73). Eventually, dopamine deficiency leads to symptoms such as rigidity, rest tremor, bradykinesia and abnormal gait, characteristic of PD. While there are existing treatments to ameliorate PD symptoms, these treatments possess several limitations such as decreased efficacy after years of usage (73-76). Hence, SCT offers PD patients an alternate treatment to not only manage the symptoms but also possibly treat the root cause of the disease. SCT aims to replace degenerated dopaminergic neurons with healthy neurons capable of producing dopamine. The pioneer of PD SCT was foetal tissues (76,77). However, due to ethical issues and lack of sources, scientists began looking for better alternatives such as MSCs and iPSCs.
The efficacy of MSCs in treating PD has been validated in preclinical studies with improved behavioural and motor improvements (20,78-80). Studies reported that umbilical cord-derived MSCs (UC-MSCs) exert neuroprotective effects but undergo uncontrollable differentiation and have low survival rates (80,81). To overcome the limitation of UC-MSCs, a preclinical study was done to evaluate the efficacy of exosomes secreted from UC-MSCs (82). These exosomes were found to increase the expression of autophagy-related proteins, therefore, increasing the clearance rate of aggregation and exerting cytoprotective effects. Recently, Schiess et al. (16) published the outcomes of the world’s first clinical trial testing the use of a single intravenous infusion of allogeneic bone marrow-derived MSCs (BM-MSCs) to treat PD. The study proved that MSC therapy is safe, tolerant, and non-immunogenic to be used for AD treatment, with no serious side effects. The study also reported that MSCs trigger an anti-inflammatory response, noted to be more pronounced in higher doses of stem cell infusion. In a recent pilot study, Shigematsu and colleagues (83) investigated the safety and efficacy of multiple infusions of autologous MSCs derived from adipose tissues in three PD patients. The patients who received five or six SCT at approximately one-month intervals reported improvements in the PD severity rating scale, MDS-UPDRS. While no adverse effects were reported during the six months, further studies are required to ascertain the safety and efficacy of multiple SCT in larger sample sizes.
Instead of using MSCs for PD SCT, Schweitzer et al. opted to use iPSCs (84). This method circumvented the need for immunosuppressants as the cell source was autologous, derived from the patient’s fibroblasts via skin biopsy. Gradual clinical changes including motor assessments and symptoms were reported after 18 to 24 months post-treatment in the patient. However, the results are deemed questionable as there was no control, and both the patient and healthcare providers were informed of the procedure. In Japan, a pre-clinical study was carried out to gauge the safety and efficacy of human iPSCs-derived dopaminergic progenitor cells in treating PD (76). As both in vitro and in vivo studies showed positive outcomes, two clinical trials (JMA-IIA00384, UMIN000033564) were approved in 2018 (20,85). However, there are no updates as the clinical trials were suspended. It is worth noting that the majority of stem cell therapies are performed with the aim to treat mild and moderate PD, where the loss of dopaminergic neurons is not substantial (16,20,84,85). Nevertheless, researchers are still looking at the possibility of using cell therapy to treat advanced PD cases. Presently, there is an ongoing clinical trial to evaluate the safety and tolerability of SCT for advanced PD (ClinicalTrials.gov Identifier: NCT04802733), set to complete in 2023.
Huntington’s disease (HD)
HD is an inherited autosomal dominant progressive neurodegenerative disorder due to loss of medium spiny neurons (86). HD is due to repeated CAG trinucleotide in the Huntingtin gene, leading to the increased levels of abnormal huntingtin protein. Affected individuals experience symptoms such as cognitive impairment, chorea (involuntary twitching), and psychiatric disturbance during mid-life. The mean survival rate of HD patients is 15–20 years. In the 2000s, pilot studies involving foetal tissues (also used for PD treatment) showed great clinical improvement in HD patients (39,87). However, it was difficult to reproduce these results due to patient heterogeneity. Hence, the focus turned to SCT with the goal to reconstitute a functional information-processing network by either replacing lost neurons or reducing the formation of toxic huntingtin protein (86). Thus far, only preclinical studies have been done to evaluate the potential of SCT in treating HD.
Among the animal studies completed is an experiment to study the effects of genetically modified human MSCs overexpressing brain-derived neurotrophic factor (BDNF) in HD mice (88), where decreased levels of BDNF were found to be associated with HD severity (89). Two strains of mice were used, YAC128 (exhibits slow onset of HD) and R6/2 (exhibits rapid onset of HD). The outcome of MSC-BDNF treatment was that while both mice strains had reduced anxiety-related behaviour and increased neurogenesis, R6/2 mice had longer survival rates. In 2019, it was reported that the combination of stem cell and gene therapy had improved the motor functions (evaluated through rotarod and grip strength) and increased the life span of HD affected mice (90). In the study, rhesus monkey neural progenitor cells, derived from iPSCs were genetically modified to decrease the expression of mutated huntingtin gene, the causative factor of HD.
As one of the reasons for brain cell death in HD is due to oxidative stress (91), the potential of UC-MSCs in decreasing oxidative-induced cell death in HD rats was investigated (92). The study revealed that the paracrine activity of UC-MSCs had not only prevented oxidative stress-induced cell death but also improved motor function and degenerated striatum in the rats. Another study revealed that human striatal progenitor cells differentiated in vitro from ESC had undergone maturation and integrated into transgenic rats’ synaptic circuits following transplantation into the corpus striatum (93). Up to two months post-transplantation (before the rats were sacrificed), improvement in sensorimotor responses were observed. Nevertheless, long-term studies are crucial to evaluate the long-term survival and circuit integration of transplanted grafts.
Using gene editing, Park and team downregulated the expression of SUPT4H1 gene (involved in nucleotide repeat expansion) in neural precursor cells (NPCs) derived from iPSCs with the aim to increase the therapeutic effect of SCT in HD treatment (94). The results showed that HD mice treated with edited NPCs had improved motor function and increased neuronal density compared to HD mice treated with non-edited NPCs, suggesting that gene-edited SCT have higher efficacy in treating mutation-associated diseases such as HD.
Spinal cord injury (SCI)
SCI is an intractable and debilitating condition caused by a primary injury to the spinal cord (most often caused by motor vehicle accidents). As a result of structural damage, inflammation would sue the damaged site to clear cellular debris and initiate tissue remodelling (95,96). However, uncontrolled inflammation would lead to secondary injuries such as haemorrhage, demyelination, axonal and neuronal neuropathy, which are the underlying cause of SCI that exacerbates the condition. Although there are treatments for SCI such as prescription of anti-inflammatory medications, spinal decompression therapy to relieve pressure around the spinal cord and supportive treatments, these treatments do not provide long-term therapeutic benefits (97). Therefore, with SCT, there is a possibility of reversing spinal cord damage, in which, regenerative SCT aims to regenerate damaged spinal cord cells to revive neural circuits and reverse paralysis while neuroprotective SCT aims to limit secondary injuries through the release of neurotrophic factors (98). As with other neurological diseases, human foetal nervous tissue grafts were used to study spinal cord regeneration in the late 1970s (99).
Currently, other sources of stem cells such as NSCs and MSCs are preferred due to the ethical issues surrounding foetal tissues. In 2020, researchers reported that mice with T10 spinal cord injury showed significantly higher neurological scores, motor response, following transplantation of three-dimensional collagen/silk fibroin scaffold (3D-CF) co-cultured with NSCs compared to control mice (100). Moreover, there was an increase in axon number and a reduction in glial scarring in this test group, which was visualised by immunofluorescence microscopy, validating the positive effects of NSC therapy in treating SCI. In another study, spinal cord-type NPCs derived from iPSCs were injected into injured mice spinal cords (101). The study revealed that marked functional improvement was seen especially in mice with mild-to-moderate lesions after NPC engraftment, demonstrating the ability of NPCs to interconnect with mice neural circuits.
Using exosomes dental pulp stem cells (DPSCs), Liu and team demonstrated the therapeutic effects of exosomes in regulating inflammatory signals in mice afflicted with SCI (4). Post-infusion results showed that DPSCs decrease reactive oxygen species (ROS) levels and subsequently reduce M1 macrophage polarization in damaged tissues by suppressing the ROS-MAPK-FκB P65 signalling pathway. Hence, further studies ought to be carried out to ascertain whether disrupting this pathway would lessen neurological deficits and disabilities due to SCI. As the safety and efficacy of NPCs derived from iPSCs in treating SCI have been previously validated in preclinical studies, Sugai and colleagues have begun laying the groundwork for the world’s first clinical trial involving iPSC-derived NPCs to treat subacute complete SCI (UMIN000035074) (102).
To investigate the paracrine effects of MSCs in reducing apoptosis, inflammation and promoting angiogenesis following T10 SCI, MSC-derived exosomes were transplanted into injured mice (103). Imaging results showed that the exosomes had successfully incorporated into the neurons at the injured spinal cord site and the mice had improved motor function and decreased lesion size after undergoing SCT. The study also discovered that microRNA-21-5p was highly expressed in the exosomes and play an important role in modulating apoptosis and motor function. In a phase 1 clinical trial, three patients with thoracic level SCI were administered autologous mucosal olfactory ensheathing cell (OECs) together with BM-MSCs via lumbar puncture to assess the safety and efficacy of the SCT (104). Post therapy results showed that there was a slight improvement in the functional ability of one of the patients while the other two patients had no improvement. While none of the patients had regained their motor skills, the transplanted cells did not cause adverse effects, establishing the safety of OECs and MSCs. Currently, the only approved stem cell-based therapy for SCI is Stemirac® which is manufactured from autologous BM-MSCs (14,105). Approved by the Government of Japan in late 2018, Stemirac® was shown to improve the functional ability of 13 SCI patients with causing adverse effects in a phase II clinical trial (106).
Amyotrophic lateral sclerosis (ALS)
ALS (also known as Lou Gehrig’s disease) is a progressive neurological disorder characterised by the degeneration of motor neurons, leading to the loss of muscle control (107). The cause of ALS is largely unknown, with 5–10% of cases identified as genetically inherited. Until now, ALS patients are only offered symptomatic treatments and pharmacological drugs, Riluzole and Edaravone, to slow down the progression of ALS and slightly prolong life expectancy (108). However, with the advancement of SCT in treating neurological disorders, there is a possibility of addressing the root cause of ALS – motor neuron degeneration. Hence, stem cells are being researched as a potential treatment for ALS patients, with the focus to either regenerate motor neurons or repair degenerating motor neurons through the release of paracrine factors, or both. In fact, there is an approved SCT for ALS treatment in South Korea named Neuronata-R® (13). The treatment involving two separate intrathecal injections of autologous BM-MSCs was shown to be safe and efficacious in slowing down the progression of ALS. There is an ongoing phase III clinical trial in Korea to confirm the safety and efficacy of this SCT (ClinicalTrial.gov Identifier: NCT04745299). The study is estimated to be completed in 2026.
As MSC-based therapy were shown to improve ALS patients’ outcomes, there are many preclinical and clinical studies on the use of MSCs as a potential therapeutic. To exemplify, in preclinical studies, ALS mice transplanted with BNDF-overexpressing UC-MSC-derived from motor neurons showed improvements in motor performances and longer survival rates when treated in the early stages of ALS (109). Unfortunately, the procedure did not delay disease onset in ALS mice who underwent SCT during the pre-symptomatic phase. Contrastingly, in a separate preclinical study, SCT involving exosomes derived from DPSCs had increased post-onset survival rate in mice treated with SCT after the onset of ALS (110). The study also showed that administration of DPSC-derived exosomes had significantly improved neuromuscular junction innervation (an early pre-symptomatic manifestation of ALS) and increased the survival rate of motor neurons in ALS mice in both early and late pre-symptomatic stages of ALS.
In a phase 2 clinical trial (ClinicalTrial.gov number NCT01363401), 64 ALS patients were divided into two groups, one group received Riluzole while the other group received Riluzole together with two intrathecal injections of autologous BM-MSCs (111). The clinical trial results showed that patients in the latter group had improved clinical scores with reduced levels of proinflammatory cytokines and elevated levels of anti-inflammatory cytokines, lasting for at least six months. Similar positive results were observed in clinical trials involving BM-MSCs to treat ALS (112-114). One of studies reported a temporary delay in ALS progression following concurrent intrathecal and intravenous administration of BM-MSCs (112) while another study noted slowed disease progression in patients with rapid ALS progression after undergoing three doses of autologous BM-MSCs over a 3-month period (113). The third study was on a patient with advanced ALS who demonstrate a significant improvement in speech, strength and gait after undergoing four transfusion cycles of highly concentrated BM-MSCs in the span of nine weeks (114). After undergoing SCT, the patient experienced fewer muscle spasms and could sleep better. Instead of MSCs derived from bone marrow, another study opted to study the effects of UC-MSCs on patients’ survival rates (115). A total of 67 ALS patients were given three doses of MSCs derived from Wharton’s jelly at intervals of every 2 months via intrathecal administration (a minimally invasive route). The results, which were compared with reference patients, showed that 31% of patients had decreased progression rate following SCT while 20% of them had increased progression rate, with the rest having no changes. The researchers observed that female patients and patients who had a positive response after the first dose had better therapeutic outcomes at the end of the study.
Multiple sclerosis (MS)
MS is a chronic autoimmune and inflammatory disease of the central nervous system (CNS) (116). MS is associated with immune system-mediated attacks of neuronal myelin sheaths by autoreactive T and B cells, leading to demyelination and eventually neuronal loss. As a result of neurodegeneration and formation of plaques in the CNS, MS patients experience specific neurological disabilities depending on the location of plaques. For instance, plaques in the optic nerve would lead to vision loss. While presently there is no cure for MS, there are available treatments to treat symptoms and control the frequency and severity of acute MS attacks (117). However, these disease-modifying treatments have severe side effects (118). Therefore, researchers have turned to stem cells, particularly MSCs, as a promising curative treatment for MS patients.
Preclinical studies involving MSCs revealed that MS mice treated with placenta-derived MSCs (PMSCs) or a high-dose of exosomes derived from PMSCs demonstrated significant improvement in motor functions compared to the control group (119). In addition, immunohistological analysis showed reduced number of degenerated oligodendrocytes and decreased myelin loss in mice treated with stem cells or exosomes. Further analysis revealed that MSC-derived exosomes drive the maturation of oligodendrocytes, and hence is a potential therapy to halt the progression of MS.
In another animal study, the effects of SCT involving NSCs derived from MSCs, and MSCs were studied in murine models of MS (120). Post-therapy analysis revealed that the stem cells exerted their beneficial effects by modulating the number of regulatory T cells and T helper 17 cells (key players in cell-mediated immune response in MS) and upregulating genes responsible for myelination and neuroprotection while downregulating genes involved in inflammation and astrogliosis (a repair system activated following CNS injuries). However, it was noted that MSC-derived NSCs were significantly more potent in ameliorating disease progression of MS compared to MSCs. As for clinical trials, the efficacy of autologous BM-MSCs therapy via intrathecal or intravenous route was examined in patients with active and progressive MS (ClinicalTrial.gov Identifier: NCT0216601) (121). The study reported that almost 50% of patients treated with SCT had no disease activity, hypothesized to be due to the immunomodulatory and neuroprotective effects of MSCs. It was also observed that intrathecal administration of MSCs was more effective in controlling MS compared to the intravenous route. However, in a phase II clinical trial, 9 MS patients showed a decline in clinical and neurophysiological measures after undergoing autologous MSC therapy, especially measures involving central nerve conduction, suggesting that the therapy fail to halt the progression of MS (122). The researchers stated that one of the reasons for the poor outcome is the severity of MS in the patients as SCT is more likely to work in less severe patients compared to those with end-stage of disease.
In another clinical trial, the efficacy of nonmyeloablative autologous HSCT in treating 110 patients with highly active and relapsing-remitting MS is compared with the traditional disease-modifying therapy (123). The aim of the HSCT is to repopulate the immune cells in the absence of costimulatory signals in a non-inflammatory environment. At the end of the five-year study, patients treated with HSCT, even those who received HSCT nearing the study completion, had delayed disease progression. Similar results have been reported by Boffa and colleagues on the long-term effects of HSCT in preventing MS patient deterioration and adequately improving patients’ quality of life without causing adverse effects (124).
Diabetes mellitus (DM)
DM is one of the most common non-communicable diseases. Type 1 diabetes mellitus (T1DM) is caused by the body’s autoimmune response that destroys insulin-producing beta cells in the pancreas while type 2 diabetes mellitus (T2DM) is caused by insulin resistance and insufficiency (125). Due to DM’s high morbidity and mortality, substantial research is done on the treatment and management of DM. The only satisfactory option available for DM patients who are unresponsive to conventional exogenous insulin treatment is to either undergo islet or pancreas cadaveric transplantation (126). However, this treatment option is challenging due to the shortage of cadaveric donors and the need for life-long immunosuppressants. Therefore, advancement in SCT allows the differentiation of pluripotent stem cells (PSCs) into insulin-producing beta-cells for DM treatment (127). Among the many studies that have progressed to clinical trials, the recent trials, predominantly involving MSCs, are summarised in Table 4.
Table 4
| DM subtype | Type of stem cells | Clinical trial identifier | Title of clinical trial | Method of treatment | Commencement of clinical trial | Status of clinical trial |
|---|---|---|---|---|---|---|
| T1DM | MSCs | NCT03920397 | Mesenchymal stem cells in patients with type 1 diabetes mellitus | Intravenous infusion of allogenic adipose derived-MSCs | 2019 | Completed |
| NCT04061746 | Cellular therapy for Type 1 diabetes using mesenchymal stem cells | Intravenous infusion of UC-MSCs | 2019 | Recruiting | ||
| ESCs | NCT04678557 | A study to evaluate safety, engraftment and efficacy of VC-01 in subjects with T1 diabetes mellitus | Subcutaneous implantation of combination product VC-01 (PEC-01 cells loaded into an Encaptra drug delivery system) | 2020 | Recruiting | |
| T2DM | MSCs | NCT03343782 | Outcomes of expanded autologous bone marrow-derived mesenchymal stem cells therapy in Type II diabetes | Transplantation of expanded autologous BM-MSCs | 2017 | Completed |
| NCT04501341 | BM-MNC (bone-marrow mononuclear cells) and UC-MSC for Type 2 diabetes mellitus patients | Intrapancreatic catherisation of bone-marrow mononuclear cells (BM-MNCs) or intravenous transfusion of UC-MSCs | 2020 | Recruiting | ||
| NCT03658655 | Stem cells from human exfoliated teeth in treatment of Type 2 diabetes | Intravenous infusion of human exfoliated teeth stem cells | 2020 | Completed | ||
| NCT04642911 | Long term follow-up of subjects with Diabetes 2 type treatment with ex vivo gene therapy using autologous mesenchymal stem cell | Intravenous infusion of autologous MSCs | 2020 | Active, not recruiting |
T1DM, type 1 diabetes mellitus; MSCs, mesenchymal stem cells; ESCs, embryonic stem cells; UC-MSCs, umbilical cord-derived MSCs; T2DM, type 2 diabetes mellitus; BM-MSCs, bone marrow-derived MSCs.
The first long-term glycaemic correction for T1DM was achieved by implanting encapsulated stem cell-derived beta cells into mice’s intraperitoneal space (21). The implantation did not evoke the immune rejection of stem cells as the encapsulation was designed to evade the immune system, avoiding the need for immunosuppressants. Until the end of the study, the mice remained normoglycemic and viable insulin-producing cells were found in the implants on the 174th day after being taken out. Thereby, there is an ongoing clinical trial (ClinicalTrials.gov Identifier: NCT04678557) involving VC-01™, an encapsulated device containing ESC differentiated pancreatic progenitor cells aiming to evaluate the products’ safeness, efficacy, and patient tolerance for 1 year in T1DM patients. The study is expected to be completed by October 2022.
On the other hand, T2DM is associated with elevated oxidative stress due to hyperglycaemia and insulin resistance which leads to hypoxia (128). A study on the viability of engineered pancreatic islets in hypoxic environments, when co-cultured with Wharton’s jelly MSCs, showed higher viability, higher vascularisation and increased anti-inflammatory cytokines compared to negative controls (129). Studies reported that insulin requirement and haemoglobin A1c (HbA1c) levels had decreased following transplantation of autologous BM-MSCs in T2DM patients (22,130). However, the effects of SCT were short-lived suggesting this treatment is more efficacious in patients with less than 10 years of T2DM and body mass index of less than 23 (131). In 2020, Hogrebe and colleagues successfully controlled the differentiation of beta cells from human ESC by manipulating the cells’ actin cytoskeleton and managed to maintain normoglycemia in severe diabetic mice after nine months of SCT (132). In addition, CRISPR-Cas 9 was employed to edit a faulty Wolfram syndrome-1 gene in patient-derived iPSCs (133). Following the transplantation of corrected differentiated beta-cells into severe diabetic mice, the mice were diabetes-free and teratoma-free. This provides a new paradigm for autologous cell replacement therapy for diabetic patients in the future by editing other diabetic-related genes.
Retinal degenerative disorders
Retinal degenerative disorders are conditions relating to the loss or dysfunction of photoreceptors and retinal pigment epithelium (RPE) cells that leads to progressive vision loss and eventually complete blindness if left untreated (134). Although retinal degenerative disorders such as age-related macular degeneration (MD), retinitis pigmentosa (RP) and Stargardt’s macular dystrophy (SMD) are the number one cause of irreversible blindness is developed countries and the second leading cause of blindness in developing countries, there is no available treatments to prevent vision loss (135) and reverse retinal degeneration. Nonetheless, SCT offers hope to patients with retinal degenerative disorders due to the ability of stem cells to differentiate in retinal cells and promote cell regeneration through paracrine signalling, treating the root cause of these disorders. To illustrate, mice with induced retinal degeneration showed increased retinal thickness and preserved retinal morphology following intravitreal injection of DPSCs compared to the control group (136). Histological analysis revealed that the DPSCs were localised at the site of injury and had integrated themselves in the photoreceptor and RPE layers. The potential of SCT as a treatment for retinal degenerative disorders is being evaluated in various clinical trials worldwide as reflected in Table 5.
Table 5
| Disease | Type of stem cells | Clinical trial identifier | Title of clinical trial | Method of treatment | Commencement of clinical trial | Status of clinical trial |
|---|---|---|---|---|---|---|
| MD | iPSCs | NCT04339764 | Autologous Transplantation of Induced Pluripotent Stem Cell-Derived Retinal Pigment Epithelium for Geographic Atrophy Associated With Age-Related Macular Degeneration | Subretinal transplantation of iPSC-derived RPE | 2020 | Recruiting |
| iPSCs | NCT05445063 | Safety and Efficacy of Autologous Transplantation of iPSC-RPE in the Treatment of Macular Degeneration | Subretinal transplantation of iPSC-derived RPE | 2022 | Recruiting | |
| RP | ESCs | NCT03963154 | Interventional Study of Implantation of hESC-derived RPE in Patients With RP Due to Monogenic Mutation | Subretinal implantation of ESC-derived RPE | 2019 | Recruiting |
| MSCs | NCT04224207 | Management of Retinitis Pigmentosa by Mesenchymal Stem Cells by Wharton’s Jelly Derived Mesenchymal Stem Cells (WJ-MSC) | Sub-tenon injection of Wharton’s Jelly-derived MSCs | 2020 | Completed | |
| MSCs | NCT04763369 | Investigation of Therapeutic Efficacy and Safety of UMSCs for the Management of Retinitis Pigmentosa (RP) | Sub-tenon or suprachoroidal injection of UC-MSCs | 2021 | Recruiting | |
| SMD | ESCs | NCT02445612 | Long Term Follow Up of Sub-retinal Transplantation of hESC Derived RPE Cells in Stargardt Macular Dystrophy Patients | Subretinal transplantation of ESC-derived RPE | 2015 | Completed |
| ESCs | NCT02941991 | A Follow up Study to Determine the Safety and Tolerability of Sub-retinal Transplantation of Human Embryonic Stem Cell Derived Retinal Pigmented Epithelial (hESC-RPE) Cells in Patients With Stargardt’s Macular Dystrophy (SMD) | Subretinal transplantation of ESC-derived RPE | 2016 | Completed |
MD, macular degeneration; iPSCs, induced pluripotent stem cells; RPE, retinal pigment epithelium; ESCs, embryonic stem cells; MSCs, mesenchymal stem cells; RP, retinitis pigmentosa; UC-MSCs, umbilical cord-derived MSCs; SMD, Stargardt macular dystrophy.
Macular degeneration (MD)
MD is associated with progressive loss of RPE cells which play a vital role in the maintenance and survival of photoreceptors (137). Due to the loss of photoreceptors, MD patients experience vision loss as the disease progresses (138). MD is extremely prevalent in developed countries and if left untreated may progress to severe central vision loss, affecting one’s quality of life. Hence, by replacing dying RPE cells with those derived from stem cells, it is possible to cure MD.
The first use of ESCs in treating MD reported that ESC transplantation is safe and feasible as there was no immune rejection nor tumour formation (139). In 2018, ESC-derived RPE cells transplantation on patients with wet MD showed improved visual acuity and regeneration of RPE cells in the damaged area (23,140). ESC-derived RPE transplantation also demonstrated positive changes in halting the progression of late-stage dry MD (24). Nevertheless, long-term efficacy of transplantation will be reported in 2024 (ClinicalTrials.gov identifier: NCT022860890).
Following the introduction of iPSCs, differentiated RPE cells derived from reprogrammed skin cells were transplanted into an MD patient (141). A few years later, it was found that the patient’s vision did not deteriorate hinting that iPSC transplantation halted AMD progression (142). A significantly lower cost of iPSC therapy was introduced on a sexagenarian man from an allogeneic donor (143). However, the efficacy of this transplantation remains unreported. Besides using PSCs as a source of RPE cells, suprachoroidal adipose-derived MSCs is also used to treat MD and severe vision acuity (144). The study reported that there was no adverse reaction in all patients and 87.5% of patients had vision improvement after one-year post-transplantation.
Retinitis pigmentosa (RP)
RP is an inherited heterogeneous condition characterised by the progressive loss of rod photoreceptors which are responsible for vision in dim light and peripheral vision (145). As a result, patients with RP experience gradually night blindness and tunnel vision as the rod cells are mainly located at the rim of the retina. In later stages of the disease, patients will completely lose their vision. To prevent this from occurring, either the progression of RP must be stopped, or the degeneration of rod photoreceptors must be reversed. Hence, SCT is an attractive potential treatment for RP patients due to the regenerative and reparative effects of stem cells.
The paracrine effects of transplanted stem cells were validated by Liu and colleagues by transplanting different types of stem cells into the subretinal space of RP mouse models (146). The study discovered that VEGF-A (vascular endothelial growth factor A) released by stem cells exerts neurotrophic and neuroprotective effects, catalysing the repair of degenerative photoreceptors cells. It was also noted that ASCs would be a better source of stem cells as they have the longest lifespan compared to other types of stem cells. In a preclinical study, the light responses in Pde6brd1 mice with autosomal recessive RP with end-stage degeneration were investigated following transplantation of human ESC-derived photoreceptors (147). The study reported that the transplanted cells had restored visual acuity, accessed through light avoidance and optomotor response test.
In a phase I clinical trial (ClinicalTrial.gov Identifier: NCT01531348), 14 patients with advanced RP were administered autologous BM-MSCs at different concentrations via the intravitreal route (148). The study stated that all patients demonstrated improved best-corrected visual acuity which lasted for a year. Moreover, the highest improvement was seen in patients who received the least dose of BM-MSCs. Although there were a few adverse reactions post-transplantation such as mild eye pain, choroidal detachment, flashes, eye synechiae and vision loss, the study concluded that BM-MSC therapy is safe. However, long-term studies involving more participants are needed to ascertain the safety of SCT. The long-term effects and survival of iPSC transplants were evaluated in Pde6b (one of the causal genes identified in RP) knockout rats (149). The study revealed that the transplanted iPSC-derived retinal cells survived and differentiated into RPE and photoreceptors in the affected area, restoring retinal function after 10 months of transplantation.
Stargardt’s macular dystrophy (SMD)
SMD is an autosomal recessive genetic disorder, largely caused by mutations in the ABCA4 (ATP-binding cassette, sub-family A, member 4) gene which disrupt the production of retina transporter protein and leads to the accumulation of lipofuscin in the RPE (150,151). Due to the loss of photoreceptors, patients with SMD develop progressive and irreversible vision loss. SMD is the most common form of macular degeneration in children and young adults. Currently, there is no cure nor treatment to halt the development of SMD. Therefore, with SCT, there is a possibility of regenerating and repairing degenerative photoreceptors with the hope of improving SMD patients’ eyesight and halting the progression of the disease.
Hence, to evaluate the safety and efficacy of ESC transplantation for the treatment of SMD, a clinical trial was carried out for three years (152). The phase I clinical trial involved three patients were showed preserved structural function of treated eye, with one of them demonstrating improved best-corrected visual acuity while the others maintained their best-corrected visual acuity, suggesting that ESC therapy is able to halt the progression of SMD. A similar study was conducted by Li and team to investigate the long-term safety and efficacy of RPE cells derived from ESCs in treating early-stage of SMD for five years (153). It was observed that all 7 patients had improved or stable visual acuity in the first few months following ESC transplantation, determined by neurophysiological examinations. However, at the end of the study, two of the patients had a decline in visual function, but one of the two was shown to be stable when compared to the corresponding untreated eye. Nevertheless, the treatment was noted to be safe as none of the patients had severe side effects.
To determine the effects of ESC-derived RPE cells on retinal structure and function, 12 patients with advanced SMD underwent subretinal stem cell transplantation (154). Results showed that the stem cells were tolerable and did not trigger inflammatory responses. However, the treatment did not halt the progression of SMD nor improve the visual acuity of the 12 patients, assessed using a microperimetry. The study outcome suggests that SCT does not work in patients with advanced SMD and severe visual impairment. In another study involving MSCs, 31 patients with SMD were treated with autologous BM-MSCs (155). The results showed that most patients had positive outcomes following SCT with 23.5% of them remaining stable throughout the study and 5% of patients demonstrated continued progression of SMD. The stem cell transplantation was deemed safe as no adverse effects were reported.
COVID-19 infections
COVID-19 is a highly infectious disease of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (156). The virus is known to attack the lungs of the infected, increasing the susceptibility to lower respiratory tract infections. The hallmark of COVID-19 is immune system dysregulation due to massive release of cytokines, leading to severe inflammation—a state known as a cytokine storm (156,157). The aftermath of cytokine storms is severe tissue damage, commonly resulting in acute lung failure associated with Acute Respiratory Distress System (ARDS), pneumonia and lung fibrosis. To combat the effects of the cytokine storm, the use of SCT as a treatment option seems logical due to its anti-inflammatory actions. In addition, SCT has been researched previously as a potential treatment for viral infections such as hepatitis B (158), dengue virus (159), human immunodeficiency virus 1 (HIV-1) (160), influenza virus (161), and lung diseases, namely chronic obstructive pulmonary disease (162) and pulmonary fibrosis (163). Therefore, SCT was one of the very first clinical trials conducted in China as a promising treatment for patients with severe COVID-19 infections (40).
Many studies opted to transplant MSCs into COVID-19 patients. It is worth noting that SCT was mostly used for critically ill COVID-19 patients unresponsive to conventional treatments in clinical studies. MSC was chosen due to its robust immunomodulatory function in preventing and ameliorating cytokine storms, and its ability in promoting tissue repair (25,164,165). Moreover, due to the lack of viral receptors such as ACE2 and TMPRSS2, transplanted MSC will not be infected by SARS-CoV-2 (164,166). A pilot study involving MSCs reported 100% improved pulmonary functions and the disappearance of cytokine storms in patients within 6 days (26). Whereas a Phase I trial of UC-MSC therapy showed 100% recovery with a decrease in COVID-19 progression biomarker (IL-6) (165). UC-MSCs transplantation was also reported as safe and effective for COVID-19 patients with ARDS (25). Following UC-MSC transplantation, the patients had a marked decrease in inflammatory cytokines and two of the patients experienced serious adverse effects. Nevertheless, the long-term effects of UC-MSC therapy were determined by Shi and colleagues in a longitudinal study involving 101 severe COVID-19 patients (ClinicalTrial.gov identifier: NCT04288102) (25). The one-year study reported that the 65 patients treated with SCT showed better recovery of lung lesions and fewer post-infection symptoms compared to the placebo group. There are studies suggesting that higher IL-6 levels enhance immunomodulatory functions of MSCs, resulting in better clinical improvement in critically ill patients treated with Wharton’s jelly MSCs with a decrease in inflammatory cytokines (165,167).
The safety and efficacy of ESCs-derived-immunity-and-matrix-regulatory cells (IMRCs) in treating lung injury and fibrosis due to COVID-19 was evaluated through in vivo studies (168). IMRCs possess enhanced immunomodulatory and anti-fibrotic effects to ameliorate lung damage (40). Nevertheless, clinical trials are required to validate the potential use of IMRCs for COVID-19 infections. Moreover, various studies reported decreased COVID-19 survival rates in individuals who have received HSCT possibly due to weakened immune systems (169-171). Interestingly, one study involving 77 subjects reported that this group of patients were able to survive COVID-19 and had a similar immune response as the general population, contradicting the above statement (172). Thus, the efficacy and role of HSCs in the cellular microenvironment must be investigated to achieve maximum therapeutic outcomes. Nevertheless, there are a number of completed SCT clinical trials with the aim of using MSCs to treat COVID-19 infections (Table 6).
Table 6
| Clinical trial identifier | Title of clinical trial | Method of treatment | Commencement of clinical trial | Status of clinical trial | Sponsor |
|---|---|---|---|---|---|
| NCT04713878 | Mesenchymal stem cells therapy in patients with COVID-19 pneumonia | Intravenous infusion of MSCs | 2021 | Completed | Kanuni Sultan Suleyman Training and Research Hospital (Istanbul) |
| NCT04473170 | Study evaluating the safety and efficacy of autologous non-hematopoietic peripheral blood stem cells in COVID-19 | Administration of stem cells via jet nebulisation | 2020 | Completed | Abu Dhabi Stem Cells Center |
| NCT04898088 | A proof of concept study for the DNA repair driven by the mesenchymal stem cells in critical COVID-19 patients | Intravenous infusion of MSCs | 2020 | Completed | SBÜ Dr. Sadi Konuk Education and Research Hospital (Istanbul) |
| NCT04625738 | Efficacy of infusions of MSC from Wharton Jelly in SARS-CoV-2 (COVID-19) related acute respiratory distress syndrome | Intravenous infusion of Wharton’s jelly MSCs | 2020 | Completed | Central Hospital, Nancy, France |
| NCT04382547 | Treatment of COVID-19 associated pneumonia with allogenic pooled olfactory mucosa-derived mesenchymal stem cells | Intravenous injection of olfactory mucosa-derived MSCs | 2020 | Completed | Institute of Biophysics and Cell Engineering of National Academy of Sciences of Belarus |
| NCT04522986 | An exploratory study of ADR-001 in patients with severe pneumonia caused by SARS-CoV-2 infection | Intravenous administration of adipose-derived MSCs | 2020 | Completed | Rohto Pharmaceutical Co., Ltd. (Japan) |
| NCT04288102 | Treatment with human umbilical cord-derived mesenchymal stem cells by severe coronavirus disease 2019 (COVID-19) | Intravenous administration of UC-MSCs | 2020 | Completed | Beijing 302 Hospital (China) |
| NCT04349631 | A clinical trial to determine the safety and efficacy of Hope biosciences autologous mesenchymal stem cell therapy (HB-adMSCs) to provide protection against COVID-19 | Intravenous infusion of autologous adipose-derived MSCs | 2020 | Completed | Hope Biosciences Stem Cell Research Foundation (USA) |
| NCT04535856 | Therapeutic study to evaluate the safety and efficacy of DW-MSC in COVID-19 patients | Intravenous administration of allogeneic MSCs | 2020 | Completed | Ina-Respond (Indonesia) |
| NCT04573270 | Mesenchymal stem cells for the treatment of COVID-19 | Intravenous injection of MSCs | 2020 | Completed | Thomas Advanced Medical LLC (USA) |
| NCT04355728 | Use of UC-MSCs for COVID-19 patients | Intravenous administration of UC-MSCs | 2020 | Completed | Camillo Ricordi (USA) |
| NCT04392778 | Clinical use of stem cells for the treatment of COVID-19 | Intravenous administration of MSCs | 2020 | Completed | SBÜ Dr. Sadi Konuk Education and Research Hospital (Istanbul) |
MSCs, mesenchymal stem cells; COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; UC-MSCs, umbilical cord-derived MSCs.
Besides using stem cells to directly treat COVID-19 infections, effective vaccines can be created by generating stem cell-like memory T cells, derived from recovering COVID-19 patients as they retain SARS-CoV-2-specific memory T cells up to 317 days post-infection (173). With this data, there is a possibility of developing an effective vaccine capable of repopulating memory and effector T cells in vaccinated individuals. Recently, a screening method was developed to test potential inhibitors of SARS-CoV-2 using ESC-derived cardiomyocytes (174). This screening method helps to accelerate the discovery of new drugs to treat cardiovascular patients with increased susceptibility to severe COVID-19 infections, especially those who have a contraindication to vaccination.
Challenges in stem cell therapy development
While the future of SCT appears bright, there are many challenges faced in validating SCT for clinical use. These challenges include uncertainty in the underlying stem cell mechanism, ethical issues, genetic instability, and potential immune rejection of transplanted stem cells.
Uncertainty in the underlying stem cell mechanism
The biggest challenge in adopting SCT as a treatment plan is the lack of understanding of the function and mechanism of stem cells in treating and ameliorating diseases. Even with years of research in the properties of stem cells, researchers are only able to postulate the workings of the stem cells in modulating inflammation and repair (175-177). Although researchers have developed techniques to differentiate stem cells into their desired cell type, the ideal manufacturing conditions and transplant methods are yet to be known. This uncertainty puts patients undergoing SCT at risk of having severe and unforeseen complications. For instance, a patient was found to have developed a mass in his spinal cord, twelve years after undergoing olfactory mucosa autograft transplantation in an approved clinical trial (178). Hence, to ensure the long-term safety of SCT, clinical trial participants must be carefully monitored in long-term follow-ups for side effects.
Ethical issues
Ethical issues involving SCT have been an ongoing debate since the introduction of ESCs. The standard method of isolating ESCs is by destroying blastocysts to obtain the inner cell mass containing stem cells (27). This method is the root of the ethical conflict as embryos, which have the potential to develop into human beings, are sacrificed, violating the dignity of human life. Wanting to solve this ethical controversy, a technique, namely blastomere biopsy, was invented to obtain ESCs without harming the embryos (179). Nonetheless, iPSCs sourced from somatic cells bypassed the ethical conundrum. However, iPSCs themselves presented another ethical problem. Owning to their ability to divide infinitely, there are several concerns regarding the potential use of iPSCs in cloning (46,180). The concerns of stakeholders are the possibility of engineering human embryos and the production of human-animal chimaeras from iPSCs. To prevent these occurrences from happening, there are laws in place worldwide prohibiting research involving human cloning (180).
Genetic instability
Genetic instability presents a challenge in using PSCs such as ESCs and iPSCs. It is said that genes associated with the properties of PSCs such as rapid proliferation rate and self-renewal are the integral components in oncogenesis, leading to tumorigenesis (181). This applies particularly in research involving iPSCs as studies reported that there is a possibility of increased oncogene expressions (182,183) as highly oncogenic transcription factors are induced in reprogrammed cells (16). Moreover, deleting the tumour suppressor gene, p53 is said to enhance the efficiency of the reprogramming process (184,185). However, this technique is a double-edged sword as the removal of p53, the gatekeeper of cellular division, will increase the likelihood of tumourigenicity in iPSCs (185). The risk of tumourigenesis is also present in ESCs (181). Furthermore, following prolonged culture, stem cells exhibit high levels of genetic instability and spontaneously develop into cancer stem cells, which contribute to tumorigenesis (186). Although differentiated stem cells will be subjected to in vivo tumourigenicity studies, the risk of tumour formation remains due to uncontrolled cell proliferation during cell culture (187). Thus, it is vital for researchers to conduct a thorough tumourigenicity assessment. Stringent standards in examining cells prior to clinical transplantation should be a routine practice among researchers to ensure SCT safety.
Immune rejection
Another obstacle in SCT is immune rejection which occurs when transplanted stem cells (usually non-autologous sources) are recognised as foreign invaders, triggering the body’s immune response to destroy these foreign cells. To avoid immune rejection, patients undergoing SCT are given immunosuppressants to suppress their immune response, consequently minimising the risk of rejection (188). However, suppresing the immune system increases the chances of developing opportunistic infections, defeating the therapeutic purpose of SCT. Hence, autologous iPSCs is a good choice due to their low immunogenicity (31). Nonetheless, autologous iPSC treatment is impractical for clinical translation as it is costly, time-consuming, riskier due to its intrinsic genetic instability and requires strict quality control (188,189). Furthermore, potential immunogenicity of iPSCs has been reported due to spontaneous mitochondrial mutations during reprogramming processes (190). Another alternative is to use MSCs, which are reported to possess immunoregulatory effects (191,192). MSCs are able to effectively repress the development of immune cells and subsequently suppress the immune system, decreasing the risk of stem cell transplant rejection. While autologous MSCs are preferred in SCT as the chances of immune rejection can be minimised, patient-derived MSCs, especially those derived from patients with autoimmune diseases are shown to be dysfunctional and non-immunosuppressive (193). Likewise, there are evidences of immune rejection following allogeneic MSC therapy (194,195). Therefore, to further enhance the immunosuppressive effects of MSCs, researchers have pre-treated MSCs with anti-inflammatory factors such as IL-7 (196) or genetically modified MSCs to increase the expression of immunomodulatory genes (197). However, the drawback of manipulating MSCs prior to transplantation is the increased risk of tumorigenesis in the long run. Hence, the best way to overcome the potential of immune rejection is by finding an approach to create an invisibility cloak for transplanted stem cells. Several researchers are looking into ways to bypass the immune system by disrupting certain immune checkpoints (198) and gene-editing stem cells to improve immune compatibility without inducing tumorigenesis (199,200).
Conclusions
SCT offers hope to patients with incurable diseases due to its potential to repair diseased organs and improve their quality of life. With the accelerated research in SCT, it will be a matter of time before researchers overcome the aforementioned challenges. Nevertheless, in the pursuit of changing the approval status of SCT, patients’ safety and ethics must remain the utmost priority. As the current focus of SCT research is to investigate its safety and efficacy, further studies should be conducted in understanding the regenerative capabilities of stem cells and the long-term effects of SCT. To ensure SCT becomes accessible to many patients, the establishment of universal cell banks is already underway. Universal cell banks aim to modulate the immunogenicity of donor cells to improve donor compatibility and decrease the chances of immune rejection. This initiative will also reduce the cost of stem cell transplantation that would otherwise be exorbitant, especially if the source is derived from the patient’s own cells. In the future, regenerative medicine will be a revolutionary treatment for all intractable diseases.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://sci.amegroups.com/article/view/10.21037/sci-2022-021/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://sci.amegroups.com/article/view/10.21037/sci-2022-021/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Tachibana A, Santoso MR, Mahmoudi M, et al. Paracrine Effects of the Pluripotent Stem Cell-Derived Cardiac Myocytes Salvage the Injured Myocardium. Circ Res 2017;121:e22-36. [Crossref] [PubMed]
- Marcozzi C, Frattini A, Borgese M, et al. Paracrine effect of human adipose-derived stem cells on lymphatic endothelial cells. Regen Med 2020;15:2085-98. [Crossref] [PubMed]
- Han C, Sun X, Liu L, et al. Exosomes and Their Therapeutic Potentials of Stem Cells. Stem Cells Int 2016;2016:7653489. [Crossref] [PubMed]
- Liu C, Hu F, Jiao G, et al. Dental pulp stem cell-derived exosomes suppress M1 macrophage polarization through the ROS-MAPK-NFκB P65 signaling pathway after spinal cord injury. J Nanobiotechnology 2022;20:65. [Crossref] [PubMed]
- Li X, Corbett AL, Taatizadeh E, et al. Challenges and opportunities in exosome research-Perspectives from biology, engineering, and cancer therapy. APL Bioeng 2019;3:011503. [Crossref] [PubMed]
- Thomas ED, Lochte HL Jr, Lu WC, et al. Intravenous infusion of bone marrow in patients receiving radiation and chemotherapy. N Engl J Med 1957;257:491-6. [Crossref] [PubMed]
- Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature 1981;292:154-6. [Crossref] [PubMed]
- Lazarus HM, Haynesworth SE, Gerson SL, et al. Ex vivo expansion and subsequent infusion of human bone marrow-derived stromal progenitor cells (mesenchymal progenitor cells): implications for therapeutic use. Bone Marrow Transplant 1995;16:557-64. [PubMed]
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts. Science 1998;282:1145-7. [Crossref] [PubMed]
- Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007;131:861-72. [Crossref] [PubMed]
- Bretzner F, Gilbert F, Baylis F, et al. Target populations for first-in-human embryonic stem cell research in spinal cord injury. Cell Stem Cell 2011;8:468-75. [Crossref] [PubMed]
- Cyranoski D. Japanese woman is first recipient of next-generation stem cells. Nature 2014; [Crossref]
- Oh KW, Moon C, Kim HY, et al. Phase I trial of repeated intrathecal autologous bone marrow-derived mesenchymal stromal cells in amyotrophic lateral sclerosis. Stem Cells Transl Med 2015;4:590-7. [Crossref] [PubMed]
- Matsushita S, Tachibana K, Kusakabe T, et al. The Roadmap to Approval under Japan's Two-Track Regulatory System: Comparing Six Regenerative Medical Products. Cell Stem Cell 2020;27:515-8. [Crossref] [PubMed]
- Nair GG, Liu JS, Russ HA, et al. Recapitulating endocrine cell clustering in culture promotes maturation of human stem-cell-derived β cells. Nat Cell Biol 2019;21:263-74. [Crossref] [PubMed]
- Schiess M, Suescun J, Doursout MF, et al. Allogeneic Bone Marrow-Derived Mesenchymal Stem Cell Safety in Idiopathic Parkinson's Disease. Mov Disord 2021;36:1825-34. [Crossref] [PubMed]
- Ljungman P, Bregni M, Brune M, et al. Allogeneic and autologous transplantation for haematological diseases, solid tumours and immune disorders: current practice in Europe 2009. Bone Marrow Transplant 2010;45:219-34. [Crossref] [PubMed]
- Cohen S, Roy J, Lachance S, et al. Hematopoietic stem cell transplantation using single UM171-expanded cord blood: a single-arm, phase 1-2 safety and feasibility study. Lancet Haematol 2020;7:e134-45. [Crossref] [PubMed]
- Kim HJ, Seo SW, Chang JW, et al. Stereotactic brain injection of human umbilical cord blood mesenchymal stem cells in patients with Alzheimer's disease dementia: A phase 1 clinical trial. Alzheimers Dement (N Y) 2015;1:95-102. [Crossref] [PubMed]
- Takahashi J. iPS cell-based therapy for Parkinson's disease: A Kyoto trial. Regen Ther 2020;13:18-22. [Crossref] [PubMed]
- Vegas AJ, Veiseh O, Gürtler M, et al. Long-term glycemic control using polymer-encapsulated human stem cell-derived beta cells in immune-competent mice. Nat Med 2016;22:306-11. [Crossref] [PubMed]
- Le PT, Phu N, Van TP, et al. A type 2 diabetes mellitus patient was successfully treated by autologous bone marrow-derived stem cell transplantation: a case report. Biomed Res Ther 2019;6:2966-9. [Crossref]
- da Cruz L, Fynes K, Georgiadis O, et al. Phase 1 clinical study of an embryonic stem cell-derived retinal pigment epithelium patch in age-related macular degeneration. Nat Biotechnol 2018;36:328-37. [Crossref] [PubMed]
- Riemann CD, Banin E, Barak A, et al. Phase I/IIa clinical trial of human embryonic stem cell (hESC)-derived retinal pigmented epithelium (RPE, OpRegen) transplantation in advanced dry form age-related macular degeneration (AMD): interim results. Investig Ophthalmol Vis Sci 2020;61:865.
- Lanzoni G, Linetsky E, Correa D, et al. Umbilical cord mesenchymal stem cells for COVID-19 acute respiratory distress syndrome: A double-blind, phase 1/2a, randomized controlled trial. Stem Cells Transl Med 2021;10:660-73. [Crossref] [PubMed]
- Shi L, Yuan X, Yao W, et al. Human mesenchymal stem cells treatment for severe COVID-19: 1-year follow-up results of a randomized, double-blind, placebo-controlled trial. EBioMedicine 2022;75:103789. [Crossref] [PubMed]
- Sherley JL. Human Embryonic Stem Cell Research: No Way Around a Scientific Bottleneck. J Biomed Biotechnol 2004;2004:71-2. [Crossref] [PubMed]
- Dever DP, Bak RO, Reinisch A, et al. CRISPR/Cas9 β-globin gene targeting in human haematopoietic stem cells. Nature 2016;539:384-9. [Crossref] [PubMed]
- Antony JS, Haque AA, Lamsfus‐Calle A, et al. CRISPR/Cas9 system: A promising technology for the treatment of inherited and neoplastic hematological diseases. Adv Cell Gene Ther 2018;1:e10. [Crossref]
- Jose J, George T, Thomas AM. Regulation of Stem Cell-Based Research in India in Comparison with the US, EU and other Asian Countries: Current Issues and Future Perspectives. Curr Stem Cell Res Ther 2020;15:492-508. [Crossref] [PubMed]
- Golchin A, Farahany TZ. Biological Products: Cellular Therapy and FDA Approved Products. Stem Cell Rev Rep 2019;15:166-75. [Crossref] [PubMed]
- Pellegrini G, Ardigò D, Milazzo G, et al. Navigating Market Authorization: The Path Holoclar Took to Become the First Stem Cell Product Approved in the European Union. Stem Cells Transl Med 2018;7:146-54. [Crossref] [PubMed]
- Gupta PK, Dutta S, Kala S, et al. Phase IV postmarketing surveillance study shows continued efficacy and safety of Stempeucel in patients with critical limb ischemia due to Buerger's disease. Stem Cells Transl Med 2021;10:1602-13. [Crossref] [PubMed]
- Chisholm J, Ruff C, Viswanathan S. Current state of Health Canada regulation for cellular and gene therapy products: potential cures on the horizon. Cytotherapy 2019;21:686-98. [Crossref] [PubMed]
- Aly RM. Current state of stem cell-based therapies: an overview. Stem Cell Investig 2020;7:8. [Crossref] [PubMed]
- Zakrzewski W, Dobrzyński M, Szymonowicz M, et al. Stem cells: past, present, and future. Stem Cell Res Ther 2019;10:68. [Crossref] [PubMed]
- Ng AP, Alexander WS. Haematopoietic stem cells: past, present and future. Cell Death Discov 2017;3:17002. [Crossref] [PubMed]
- Pan G, Mu Y, Hou L, et al. Examining the therapeutic potential of various stem cell sources for differentiation into insulin-producing cells to treat diabetes. Ann Endocrinol (Paris) 2019;80:47-53. [Crossref] [PubMed]
- Bachoud-Lévi AC, Massart R, Rosser A. Cell therapy in Huntington's disease: Taking stock of past studies to move the field forward. Stem Cells 2021;39:144-55. [Crossref] [PubMed]
- Li Z, Niu S, Guo B, et al. Stem cell therapy for COVID-19, ARDS and pulmonary fibrosis. Cell Prolif 2020;53:e12939. [Crossref] [PubMed]
- Brons IG, Smithers LE, Trotter MW, et al. Derivation of pluripotent epiblast stem cells from mammalian embryos. Nature 2007;448:191-5. [Crossref] [PubMed]
- Iaquinta MR, Mazzoni E, Bononi I, et al. Adult Stem Cells for Bone Regeneration and Repair. Front Cell Dev Biol 2019;7:268. [Crossref] [PubMed]
- Larijani B, Esfahani EN, Amini P, et al. Stem cell therapy in treatment of different diseases. Acta Med Iran 2012;50:79-96. [PubMed]
- Bongso A, Lee E. Stem cells: their definition, classification and sources. Stem Cells 2005;1-13.
- Gorecka J, Kostiuk V, Fereydooni A, et al. The potential and limitations of induced pluripotent stem cells to achieve wound healing. Stem Cell Res Ther 2019;10:87. [Crossref] [PubMed]
- Volarevic V, Markovic BS, Gazdic M, et al. Ethical and Safety Issues of Stem Cell-Based Therapy. Int J Med Sci 2018;15:36-45. [Crossref] [PubMed]
- Mushahary D, Spittler A, Kasper C, et al. Isolation, cultivation, and characterization of human mesenchymal stem cells. Cytometry A 2018;93:19-31. [Crossref] [PubMed]
- Morin V. Stem-cell replication to treat blood diseases. CMAJ 2014;186:E648. [Crossref] [PubMed]
- Dumont-Lagacé M, Li Q, Tanguay M, et al. UM171-Expanded Cord Blood Transplants Support Robust T Cell Reconstitution with Low Rates of Severe Infections. Transplant Cell Ther 2021;27:76.e1-76.e9. [Crossref] [PubMed]
- Chagraoui J, Girard S, Spinella JF, et al. UM171 Preserves Epigenetic Marks that Are Reduced in Ex Vivo Culture of Human HSCs via Potentiation of the CLR3-KBTBD4 Complex. Cell Stem Cell 2021;28:48-62.e6. [Crossref] [PubMed]
- Gluckman E, Cappelli B, Bernaudin F, et al. Sickle cell disease: an international survey of results of HLA-identical sibling hematopoietic stem cell transplantation. Blood 2017;129:1548-56. [Crossref] [PubMed]
- Baronciani D, Angelucci E, Potschger U, et al. Hemopoietic stem cell transplantation in thalassemia: a report from the European Society for Blood and Bone Marrow Transplantation Hemoglobinopathy Registry, 2000-2010. Bone Marrow Transplant 2016;51:536-41. [Crossref] [PubMed]
- Akinsheye I, Alsultan A, Solovieff N, et al. Fetal hemoglobin in sickle cell anemia. Blood 2011;118:19-27. [Crossref] [PubMed]
- Frangoul H, Altshuler D, Cappellini MD, et al. CRISPR-Cas9 Gene Editing for Sickle Cell Disease and β-Thalassemia. N Engl J Med 2021;384:252-60. [Crossref] [PubMed]
- Lattanzi A, Camarena J, Lahiri P, et al. Development of β-globin gene correction in human hematopoietic stem cells as a potential durable treatment for sickle cell disease. Sci Transl Med 2021;13:eabf2444. [Crossref] [PubMed]
- Barwick BG, Gupta VA, Vertino PM, et al. Cell of Origin and Genetic Alterations in the Pathogenesis of Multiple Myeloma. Front Immunol 2019;10:1121. [Crossref] [PubMed]
- Laubach J, Garderet L, Mahindra A, et al. Management of relapsed multiple myeloma: recommendations of the International Myeloma Working Group. Leukemia 2016;30:1005-17. [Crossref] [PubMed]
- Moreau P, San Miguel J, Ludwig H, et al. Multiple myeloma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013;24:vi133-7. [Crossref] [PubMed]
- Vaxman I, Visram A, Kumar S, et al. Autologous stem cell transplantation for multiple myeloma patients aged ≥ 75 treated with novel agents. Bone Marrow Transplant 2021;56:1144-50. [Crossref] [PubMed]
- Szmania S, Lapteva N, Garg T, et al. Ex vivo-expanded natural killer cells demonstrate robust proliferation in vivo in high-risk relapsed multiple myeloma patients. J Immunother 2015;38:24-36. [Crossref] [PubMed]
- Yin X, Tang L, Fan F, et al. Allogeneic stem-cell transplantation for multiple myeloma: a systematic review and meta-analysis from 2007 to 2017. Cancer Cell Int 2018;18:62. [Crossref] [PubMed]
- Shah N, Li L, McCarty J, et al. Phase I study of cord blood-derived natural killer cells combined with autologous stem cell transplantation in multiple myeloma. Br J Haematol 2017;177:457-66. [Crossref] [PubMed]
- Chen YA, Lu CH, Ke CC, et al. Mesenchymal Stem Cell-Derived Exosomes Ameliorate Alzheimer's Disease Pathology and Improve Cognitive Deficits. Biomedicines 2021;9:594. [Crossref] [PubMed]
- FDA grants accelerated approval for Alzheimer’s Drug [Internet]. U.S. Food and Drug Administration. 2021 [cited 31 August 2021]. Available online: https://www.fda.gov/news-events/press-announcements/fda-grants-accelerated-approval-alzheimers-drug
- Zhao X, Li D, Zhang L, et al. Mesenchymal stem cell therapies for Alzheimer's disease: preclinical studies. Metab Brain Dis 2021;36:1687-95. [Crossref] [PubMed]
- Santamaria G, Brandi E, Vitola P, et al. Intranasal delivery of mesenchymal stem cell secretome repairs the brain of Alzheimer's mice. Cell Death Differ 2021;28:203-18. [Crossref] [PubMed]
- McGinley LM, Kashlan ON, Bruno ES, et al. Human neural stem cell transplantation improves cognition in a murine model of Alzheimer's disease. Sci Rep 2018;8:14776. [Crossref] [PubMed]
- Poltavtseva RA, Samokhin AN, Bobkova NV, et al. Effect of Transplantation of Neural Stem and Progenitor Cells on Memory in Animals with Alzheimer's Type Neurodegeneration. Bull Exp Biol Med 2020;168:589-96. [Crossref] [PubMed]
- Cui GH, Guo HD, Li H, et al. RVG-modified exosomes derived from mesenchymal stem cells rescue memory deficits by regulating inflammatory responses in a mouse model of Alzheimer's disease. Immun Ageing 2019;16:10. [Crossref] [PubMed]
- Ding M, Shen Y, Wang P, et al. Exosomes Isolated From Human Umbilical Cord Mesenchymal Stem Cells Alleviate Neuroinflammation and Reduce Amyloid-Beta Deposition by Modulating Microglial Activation in Alzheimer's Disease. Neurochem Res 2018;43:2165-77. [Crossref] [PubMed]
- GBD 2016 Neurology Collaborators. Global, regional, and national burden of neurological disorders, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 2019;18:459-80. [Crossref] [PubMed]
- Surmeier DJ. Determinants of dopaminergic neuron loss in Parkinson's disease. FEBS J 2018;285:3657-68. [Crossref] [PubMed]
- Poewe W, Seppi K, Tanner CM, et al. Parkinson disease. Nat Rev Dis Primers 2017;3:17013. [Crossref] [PubMed]
- Xiao Z, Lei T, Liu Y, et al. The potential therapy with dental tissue-derived mesenchymal stem cells in Parkinson's disease. Stem Cell Res Ther 2021;12:5. [Crossref] [PubMed]
- Fan Y. Winanto, Ng SY. Replacing what's lost: a new era of stem cell therapy for Parkinson's disease. Transl Neurodegener 2020;9:2. [Crossref] [PubMed]
- Parmar M, Grealish S, Henchcliffe C. The future of stem cell therapies for Parkinson disease. Nat Rev Neurosci 2020;21:103-15. [Crossref] [PubMed]
- Bjorklund A, Kordower JH. Cell therapy for Parkinson's disease: what next? Mov Disord 2013;28:110-5. [Crossref] [PubMed]
- Gugliandolo A, Bramanti P, Mazzon E. Mesenchymal stem cell therapy in Parkinson's disease animal models. Curr Res Transl Med 2017;65:51-60. [Crossref] [PubMed]
- Farhadi M, Boroujeni ME, Kamrava SK, et al. Implantation of human olfactory ecto-mesenchymal stem cells restores locomotion in a rat model of Parkinson's disease. J Chem Neuroanat 2021;114:101961. [Crossref] [PubMed]
- Marques CR, Marote A, Mendes-Pinheiro B, et al. Cell secretome based approaches in Parkinson's disease regenerative medicine. Expert Opin Biol Ther 2018;18:1235-45. [Crossref] [PubMed]
- Li Q, Wang Y, Deng Z. Pre-conditioned mesenchymal stem cells: a better way for cell-based therapy. Stem Cell Res Ther 2013;4:63. [Crossref] [PubMed]
- Chen HX, Liang FC, Gu P, et al. Exosomes derived from mesenchymal stem cells repair a Parkinson's disease model by inducing autophagy. Cell Death Dis 2020;11:288. [Crossref] [PubMed]
- Shigematsu K, Komori N, Tahara K, et al. Repeated infusion of autologous adipose tissue-derived stem cells for Parkinson's disease. Acta Neurol Scand 2022;145:119-22. [Crossref] [PubMed]
- Schweitzer JS, Song B, Herrington TM, et al. Personalized iPSC-Derived Dopamine Progenitor Cells for Parkinson's Disease. N Engl J Med 2020;382:1926-32. [Crossref] [PubMed]
- Doi D, Magotani H, Kikuchi T, et al. Pre-clinical study of induced pluripotent stem cell-derived dopaminergic progenitor cells for Parkinson's disease. Nat Commun 2020;11:3369. [Crossref] [PubMed]
- Ross CA, Tabrizi SJ. Huntington's disease: from molecular pathogenesis to clinical treatment. Lancet Neurol 2011;10:83-98. [Crossref] [PubMed]
- Bachoud-Lévi AC. From open to large-scale randomized cell transplantation trials in Huntington's disease: Lessons from the multicentric intracerebral grafting in Huntington's disease trial (MIG-HD) and previous pilot studies. Prog Brain Res 2017;230:227-61. [Crossref] [PubMed]
- Pollock K, Dahlenburg H, Nelson H, et al. Human Mesenchymal Stem Cells Genetically Engineered to Overexpress Brain-derived Neurotrophic Factor Improve Outcomes in Huntington's Disease Mouse Models. Mol Ther 2016;24:965-77. [Crossref] [PubMed]
- Canals JM, Pineda JR, Torres-Peraza JF, et al. Brain-derived neurotrophic factor regulates the onset and severity of motor dysfunction associated with enkephalinergic neuronal degeneration in Huntington's disease. J Neurosci 2004;24:7727-39. [Crossref] [PubMed]
- Cho IK, Hunter CE, Ye S, et al. Combination of stem cell and gene therapy ameliorates symptoms in Huntington's disease mice. NPJ Regen Med 2019;4:7. [Crossref] [PubMed]
- Kumar A, Ratan RR. Oxidative Stress and Huntington's Disease: The Good, The Bad, and The Ugly. J Huntingtons Dis 2016;5:217-37. [Crossref] [PubMed]
- Ebrahimi MJ, Aliaghaei A, Boroujeni ME, et al. Human Umbilical Cord Matrix Stem Cells Reverse Oxidative Stress-Induced Cell Death and Ameliorate Motor Function and Striatal Atrophy in Rat Model of Huntington Disease. Neurotox Res 2018;34:273-84. [Crossref] [PubMed]
- Besusso D, Schellino R, Boido M, et al. Stem Cell-Derived Human Striatal Progenitors Innervate Striatal Targets and Alleviate Sensorimotor Deficit in a Rat Model of Huntington Disease. Stem Cell Reports 2020;14:876-91. [Crossref] [PubMed]
- Park HJ, Han A, Kim JY, et al. SUPT4H1-edited stem cell therapy rescues neuronal dysfunction in a mouse model for Huntington's disease. NPJ Regen Med 2022;7:8. Correction in NPJ Regen Med 2022;7:27. [Crossref] [PubMed]
- Tator CH, Fehlings MG. Review of the secondary injury theory of acute spinal cord trauma with emphasis on vascular mechanisms. J Neurosurg 1991;75:15-26. [Crossref] [PubMed]
- Pelisch N, Rosas Almanza J, Stehlik KE, et al. CCL3 contributes to secondary damage after spinal cord injury. J Neuroinflammation 2020;17:362. [Crossref] [PubMed]
- Venkatesh K, Ghosh SK, Mullick M, et al. Spinal cord injury: pathophysiology, treatment strategies, associated challenges, and future implications. Cell Tissue Res 2019;377:125-51. [Crossref] [PubMed]
- Badner A, Siddiqui AM, Fehlings MG. Spinal cord injuries: how could cell therapy help? Expert Opin Biol Ther 2017;17:529-41. [Crossref] [PubMed]
- Björklund A, Stenevi U. Intracerebral neural implants: neuronal replacement and reconstruction of damaged circuitries. Annu Rev Neurosci 1984;7:279-308. [Crossref] [PubMed]
- Jiang JP, Liu XY, Zhao F, et al. Three-dimensional bioprinting collagen/silk fibroin scaffold combined with neural stem cells promotes nerve regeneration after spinal cord injury. Neural Regen Res 2020;15:959-68. [Crossref] [PubMed]
- Kajikawa K, Imaizumi K, Shinozaki M, et al. Cell therapy for spinal cord injury by using human iPSC-derived region-specific neural progenitor cells. Mol Brain 2020;13:120. [Crossref] [PubMed]
- Sugai K, Sumida M, Shofuda T, et al. First-in-human clinical trial of transplantation of iPSC-derived NS/PCs in subacute complete spinal cord injury: Study protocol. Regen Ther 2021;18:321-33. [Crossref] [PubMed]
- Zhou X, Chu X, Yuan H, et al. Mesenchymal stem cell derived EVs mediate neuroprotection after spinal cord injury in rats via the microRNA-21-5p/FasL gene axis. Biomed Pharmacother 2019;115:108818. [Crossref] [PubMed]
- Zamani H, Soufizomorrod M, Oraee-Yazdani S, et al. Safety and feasibility of autologous olfactory ensheathing cell and bone marrow mesenchymal stem cell co-transplantation in chronic human spinal cord injury: a clinical trial. Spinal Cord 2022;60:63-70. [Crossref] [PubMed]
- Miyamoto S. Japan responds: stem-cell therapy justified. Nature 2019;569:40-1. [Crossref] [PubMed]
- Honmou O, Yamashita T, Morita T, et al. Intravenous infusion of auto serum-expanded autologous mesenchymal stem cells in spinal cord injury patients: 13 case series. Clin Neurol Neurosurg 2021;203:106565. [Crossref] [PubMed]
- Zinman L, Cudkowicz M. Emerging targets and treatments in amyotrophic lateral sclerosis. Lancet Neurol 2011;10:481-90. [Crossref] [PubMed]
- Jaiswal MK. Riluzole and edaravone: A tale of two amyotrophic lateral sclerosis drugs. Med Res Rev 2019;39:733-48. [Crossref] [PubMed]
- Wang J, Hu W, Feng Z, et al. BDNF-overexpressing human umbilical cord mesenchymal stem cell-derived motor neurons improve motor function and prolong survival in amyotrophic lateral sclerosis mice. Neurol Res 2021;43:199-209. [Crossref] [PubMed]
- Wang J, Zuzzio K, Walker CL. Systemic Dental Pulp Stem Cell Secretome Therapy in a Mouse Model of Amyotrophic Lateral Sclerosis. Brain Sci 2019;9:165. [Crossref] [PubMed]
- Oh KW, Noh MY, Kwon MS, et al. Repeated Intrathecal Mesenchymal Stem Cells for Amyotrophic Lateral Sclerosis. Ann Neurol 2018;84:361-73. [Crossref] [PubMed]
- Tavakol-Afshari J, Boroumand AR, Farkhad NK, et al. Safety and efficacy of bone marrow derived-mesenchymal stem cells transplantation in patients with amyotrophic lateral sclerosis. Regen Ther 2021;18:268-74. [Crossref] [PubMed]
- Siwek T, Jezierska-Woźniak K, Maksymowicz S, et al. Repeat Administration of Bone Marrow-Derived Mesenchymal Stem Cells for Treatment of Amyotrophic Lateral Sclerosis. Med Sci Monit 2020;26:e927484. [Crossref] [PubMed]
- Crose JJ. Treating amyotrophic lateral sclerosis with a bone marrow derived mesenchymal stem cell extracellular vesicles-A case report. IJSRA 2021;2:167-71.
- Barczewska M, Maksymowicz S, Zdolińska-Malinowska I, et al. Umbilical Cord Mesenchymal Stem Cells in Amyotrophic Lateral Sclerosis: an Original Study. Stem Cell Rev Rep 2020;16:922-32. [Crossref] [PubMed]
- Lazibat I, Rubinić Majdak M, Županić S. Multiple Sclerosis: New Aspects of Immunopathogenesis. Acta Clin Croat 2018;57:352-61. [Crossref] [PubMed]
- Garg N, Smith TW. An update on immunopathogenesis, diagnosis, and treatment of multiple sclerosis. Brain Behav 2015;5:e00362. [Crossref] [PubMed]
- Comi G, Radaelli M, Soelberg Sørensen P. Evolving concepts in the treatment of relapsing multiple sclerosis. Lancet 2017;389:1347-56. [Crossref] [PubMed]
- Clark K, Zhang S, Barthe S, et al. Placental Mesenchymal Stem Cell-Derived Extracellular Vesicles Promote Myelin Regeneration in an Animal Model of Multiple Sclerosis. Cells 2019;8:1497. [Crossref] [PubMed]
- Brown C, McKee C, Halassy S, et al. Neural stem cells derived from primitive mesenchymal stem cells reversed disease symptoms and promoted neurogenesis in an experimental autoimmune encephalomyelitis mouse model of multiple sclerosis. Stem Cell Res Ther 2021;12:499. [Crossref] [PubMed]
- Petrou P, Kassis I, Levin N, et al. Beneficial effects of autologous mesenchymal stem cell transplantation in active progressive multiple sclerosis. Brain 2020;143:3574-88. [Crossref] [PubMed]
- Tremblay F, Ansari Y, Remaud A, et al. Neurophysiological outcomes following mesenchymal stem cell therapy in multiple sclerosis. Clin Neurophysiol 2022;136:69-81. [Crossref] [PubMed]
- Burt RK, Balabanov R, Burman J, et al. Effect of Nonmyeloablative Hematopoietic Stem Cell Transplantation vs Continued Disease-Modifying Therapy on Disease Progression in Patients With Relapsing-Remitting Multiple Sclerosis: A Randomized Clinical Trial. JAMA 2019;321:165-74. [Crossref] [PubMed]
- Boffa G, Massacesi L, Inglese M, et al. Long-Term Clinical Outcomes of Hematopoietic Stem Cell Transplantation in Multiple Sclerosis. Neurology 2021. [Epub ahead of print]. pii:
10.1212/WNL.0000000000011461 . doi:10.1212/WNL.0000000000011461 .10.1212/WNL.0000000000011461 - Giovenzana A, Carnovale D, Phillips B, et al. Neutrophils and their role in the aetiopathogenesis of type 1 and type 2 diabetes. Diabetes Metab Res Rev 2022;38:e3483. [Crossref] [PubMed]
- Pomposelli T, Schuetz C, Wang P, et al. A Strategy to Simultaneously Cure Type 1 Diabetes and Diabetic Nephropathy by Transplant of Composite Islet-Kidney Grafts. Front Endocrinol (Lausanne) 2021;12:632605. [Crossref] [PubMed]
- Dor Y, Brown J, Martinez OI, et al. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature 2004;429:41-6. [Crossref] [PubMed]
- Aouacheri O, Saka S, Krim M, et al. The investigation of the oxidative stress-related parameters in type 2 diabetes mellitus. Can J Diabetes 2015;39:44-9. [Crossref] [PubMed]
- Chandravanshi B, Bhonde RR. Shielding Engineered Islets With Mesenchymal Stem Cells Enhance Survival Under Hypoxia. J Cell Biochem 2017;118:2672-83. [Crossref] [PubMed]
- Bhansali S, Dutta P, Kumar V, et al. Efficacy of Autologous Bone Marrow-Derived Mesenchymal Stem Cell and Mononuclear Cell Transplantation in Type 2 Diabetes Mellitus: A Randomized, Placebo-Controlled Comparative Study. Stem Cells Dev 2017;26:471-81. [Crossref] [PubMed]
- Nguyen LT, Hoang DM, Nguyen KT, et al. Type 2 diabetes mellitus duration and obesity alter the efficacy of autologously transplanted bone marrow-derived mesenchymal stem/stromal cells. Stem Cells Transl Med 2021;10:1266-78. [Crossref] [PubMed]
- Hogrebe NJ, Augsornworawat P, Maxwell KG, et al. Targeting the cytoskeleton to direct pancreatic differentiation of human pluripotent stem cells. Nat Biotechnol 2020;38:460-70. [Crossref] [PubMed]
- Maxwell KG, Augsornworawat P, Velazco-Cruz L, et al. Gene-edited human stem cell-derived β cells from a patient with monogenic diabetes reverse preexisting diabetes in mice. Sci Transl Med 2020;12:eaax9106. [Crossref] [PubMed]
- Veleri S, Lazar CH, Chang B, et al. Biology and therapy of inherited retinal degenerative disease: insights from mouse models. Dis Model Mech 2015;8:109-29. [Crossref] [PubMed]
- Thapa R, Khanal S, Tan HS, et al. Prevalence, Pattern and Risk Factors of Retinal Diseases Among an Elderly Population in Nepal: The Bhaktapur Retina Study. Clin Ophthalmol 2020;14:2109-18. [PubMed]
- Alsaeedi HA, Koh AE, Lam C, et al. Dental pulp stem cells therapy overcome photoreceptor cell death and protects the retina in a rat model of sodium iodate-induced retinal degeneration. J Photochem Photobiol B 2019;198:111561. [Crossref] [PubMed]
- Geng Z, Walsh PJ, Truong V, et al. Generation of retinal pigmented epithelium from iPSCs derived from the conjunctiva of donors with and without age related macular degeneration. PLoS One 2017;12:e0173575. [Crossref] [PubMed]
- Heesterbeek TJ, Lorés-Motta L, Hoyng CB, et al. Risk factors for progression of age-related macular degeneration. Ophthalmic Physiol Opt 2020;40:140-70. [Crossref] [PubMed]
- Schwartz SD, Hubschman JP, Heilwell G, et al. Embryonic stem cell trials for macular degeneration: a preliminary report. Lancet 2012;379:713-20. [Crossref] [PubMed]
- Liu Y, Xu HW, Wang L, et al. Human embryonic stem cell-derived retinal pigment epithelium transplants as a potential treatment for wet age-related macular degeneration. Cell Discov 2018;4:50. [Crossref] [PubMed]
- Mandai M, Kurimoto Y, Takahashi M. Autologous Induced Stem-Cell-Derived Retinal Cells for Macular Degeneration. N Engl J Med 2017;377:792-3. [Crossref] [PubMed]
- Takagi S, Mandai M, Gocho K, et al. Evaluation of Transplanted Autologous Induced Pluripotent Stem Cell-Derived Retinal Pigment Epithelium in Exudative Age-Related Macular Degeneration. Ophthalmol Retina 2019;3:850-9. [Crossref] [PubMed]
- Cyranoski D. Japanese man is first to receive 'reprogrammed' stem cells from another person. Nature 2017; [Crossref]
- Kahraman NS, Gonen ZB, Sevim DG, et al. First Year Results of Suprachoroidal Adipose Tissue Derived Mesenchymal Stem Cell Implantation in Degenerative Macular Diseases. Int J Stem Cells 2021;14:47-57. [Crossref] [PubMed]
- Gagliardi G, Ben M'Barek K, Goureau O. Photoreceptor cell replacement in macular degeneration and retinitis pigmentosa: A pluripotent stem cell-based approach. Prog Retin Eye Res 2019;71:1-25. [Crossref] [PubMed]
- Liu X, Chen F, Chen Y, et al. Paracrine effects of intraocularly implanted cells on degenerating retinas in mice. Stem Cell Res Ther 2020;11:142. [Crossref] [PubMed]
- Zerti D, Hilgen G, Dorgau B, et al. Transplanted pluripotent stem cell-derived photoreceptor precursors elicit conventional and unusual light responses in mice with advanced retinal degeneration. Stem Cells 2021;39:882-96. [Crossref] [PubMed]
- Tuekprakhon A, Sangkitporn S, Trinavarat A, et al. Intravitreal autologous mesenchymal stem cell transplantation: a non-randomized phase I clinical trial in patients with retinitis pigmentosa. Stem Cell Res Ther 2021;12:52. [Crossref] [PubMed]
- Yang JM, Chung S, Yun K, et al. Long-term effects of human induced pluripotent stem cell-derived retinal cell transplantation in Pde6b knockout rats. Exp Mol Med 2021;53:631-42. [Crossref] [PubMed]
- Fujinami K, Zernant J, Chana RK, et al. Clinical and molecular characteristics of childhood-onset Stargardt disease. Ophthalmology 2015;122:326-34. [Crossref] [PubMed]
- Lu LJ, Liu J, Adelman RA. Novel therapeutics for Stargardt disease. Graefes Arch Clin Exp Ophthalmol 2017;255:1057-62. [Crossref] [PubMed]
- Sung Y, Lee MJ, Choi J, et al. Long-term safety and tolerability of subretinal transplantation of embryonic stem cell-derived retinal pigment epithelium in Asian Stargardt disease patients. Br J Ophthalmol 2021;105:829-37. [Crossref] [PubMed]
- Li SY, Liu Y, Wang L, et al. A phase I clinical trial of human embryonic stem cell-derived retinal pigment epithelial cells for early-stage Stargardt macular degeneration: 5-years' follow-up. Cell Prolif 2021;54:e13100. [Crossref] [PubMed]
- Mehat MS, Sundaram V, Ripamonti C, et al. Transplantation of Human Embryonic Stem Cell-Derived Retinal Pigment Epithelial Cells in Macular Degeneration. Ophthalmology 2018;125:1765-75. [Crossref] [PubMed]
- Weiss JN, Levy S. Stem Cell Ophthalmology Treatment Study (SCOTS): Bone Marrow-Derived Stem Cells in the Treatment of Stargardt Disease. Medicines (Basel) 2021;8:10. [Crossref] [PubMed]
- Girija ASS, Shankar EM, Larsson M. Could SARS-CoV-2-Induced Hyperinflammation Magnify the Severity of Coronavirus Disease (CoViD-19) Leading to Acute Respiratory Distress Syndrome? Front Immunol 2020;11:1206. [Crossref] [PubMed]
- Song N, Wakimoto H, Rossignoli F, et al. Mesenchymal stem cell immunomodulation: In pursuit of controlling COVID-19 related cytokine storm. Stem Cells 2021;39:707-22. [Crossref] [PubMed]
- Wu Y, Huang H, Luo Y. Management of Hepatitis B Virus in Allogeneic Hematopoietic Stem Cell Transplantation. Front Immunol 2020;11:610500. [Crossref] [PubMed]
- Sakinah S, Priya SP, Mok PL, et al. Stem Cell Therapy in Dengue Virus-Infected BALB/C Mice Improves Hepatic Injury. Front Cell Dev Biol 2021;9:637270. [Crossref] [PubMed]
- Gupta RK, Abdul-Jawad S, McCoy LE, et al. HIV-1 remission following CCR5Δ32/Δ32 haematopoietic stem-cell transplantation. Nature 2019;568:244-8. [Crossref] [PubMed]
- Khatri M, Richardson LA, Meulia T. Mesenchymal stem cell-derived extracellular vesicles attenuate influenza virus-induced acute lung injury in a pig model. Stem Cell Res Ther 2018;9:17. [Crossref] [PubMed]
- Nguyen TT, Phan PT, Nguyen BH, et al. Autologous adipose-derived stem cells therapy in COPD treatment: a case report. Respirol Case Rep 2021;9:e00748. [Crossref] [PubMed]
- Li DY, Li RF, Sun DX, et al. Mesenchymal stem cell therapy in pulmonary fibrosis: a meta-analysis of preclinical studies. Stem Cell Res Ther 2021;12:461. [Crossref] [PubMed]
- Leng Z, Zhu R, Hou W, et al. Transplantation of ACE2- Mesenchymal Stem Cells Improves the Outcome of Patients with COVID-19 Pneumonia. Aging Dis 2020;11:216-28. [Crossref] [PubMed]
- Meng F, Xu R, Wang S, et al. Human umbilical cord-derived mesenchymal stem cell therapy in patients with COVID-19: a phase 1 clinical trial. Signal Transduct Target Ther 2020;5:172. [Crossref] [PubMed]
- Avanzini MA, Mura M, Percivalle E, et al. Human mesenchymal stromal cells do not express ACE2 and TMPRSS2 and are not permissive to SARS-CoV-2 infection. Stem Cells Transl Med 2021;10:636-42. [Crossref] [PubMed]
- Saleh M, Vaezi AA, Aliannejad R, et al. Cell therapy in patients with COVID-19 using Wharton's jelly mesenchymal stem cells: a phase 1 clinical trial. Stem Cell Res Ther 2021;12:410. [Crossref] [PubMed]
- Wu J, Song D, Li Z, et al. Immunity-and-matrix-regulatory cells derived from human embryonic stem cells safely and effectively treat mouse lung injury and fibrosis. Cell Res 2020;30:794-809. [Crossref] [PubMed]
- Sharma A, Bhatt NS, St Martin A, et al. Clinical characteristics and outcomes of COVID-19 in haematopoietic stem-cell transplantation recipients: an observational cohort study. Lancet Haematol 2021;8:e185-93. [Crossref] [PubMed]
- Sahu KK, Siddiqui AD, Cerny J. COVID-19 pandemic and impact on hematopoietic stem cell transplantation. Bone Marrow Transplant 2020;55:2193-5. [Crossref] [PubMed]
- Malard F, Genthon A, Brissot E, et al. COVID-19 outcomes in patients with hematologic disease. Bone Marrow Transplant 2020;55:2180-4. [Crossref] [PubMed]
- Shah GL, DeWolf S, Lee YJ, et al. Favorable outcomes of COVID-19 in recipients of hematopoietic cell transplantation. J Clin Invest 2020;130:6656-67. [Crossref] [PubMed]
- Jung JH, Rha MS, Sa M, et al. SARS-CoV-2-specific T cell memory is sustained in COVID-19 convalescent patients for 10 months with successful development of stem cell-like memory T cells. Nat Commun 2021;12:4043. [Crossref] [PubMed]
- Williams TL, Colzani MT, Macrae RGC, et al. Human embryonic stem cell-derived cardiomyocyte platform screens inhibitors of SARS-CoV-2 infection. Commun Biol 2021;4:926. [Crossref] [PubMed]
- Robey P. "Mesenchymal stem cells": fact or fiction, and implications in their therapeutic use. F1000Res 2017;6:F1000 Faculty Rev-524.
- Golchin A, Seyedjafari E, Ardeshirylajimi A. Mesenchymal Stem Cell Therapy for COVID-19: Present or Future. Stem Cell Rev Rep 2020;16:427-33. [Crossref] [PubMed]
- Liu LP, Li YM, Guo NN, et al. Therapeutic Potential of Patient iPSC-Derived iMelanocytes in Autologous Transplantation. Cell Rep 2019;27:455-466.e5. [Crossref] [PubMed]
- Woodworth CF, Jenkins G, Barron J, et al. Intramedullary cervical spinal mass after stem cell transplantation using an olfactory mucosal cell autograft. CMAJ 2019;191:E761-4. [Crossref] [PubMed]
- Chung Y, Klimanskaya I, Becker S, et al. Human embryonic stem cell lines generated without embryo destruction. Cell Stem Cell 2008;2:113-7. [Crossref] [PubMed]
- Moradi S, Mahdizadeh H, Šarić T, et al. Research and therapy with induced pluripotent stem cells (iPSCs): social, legal, and ethical considerations. Stem Cell Res Ther 2019;10:341. [Crossref] [PubMed]
- Lee AS, Tang C, Rao MS, et al. Tumorigenicity as a clinical hurdle for pluripotent stem cell therapies. Nat Med 2013;19:998-1004. [Crossref] [PubMed]
- Ou M, Zhao M, Li C, et al. Single-cell sequencing reveals the potential oncogenic expression atlas of human iPSC-derived cardiomyocytes. Biol Open 2021;10:bio053348. [Crossref] [PubMed]
- Iida T, Iwanami A, Sanosaka T, et al. Whole-Genome DNA Methylation Analyses Revealed Epigenetic Instability in Tumorigenic Human iPS Cell-Derived Neural Stem/Progenitor Cells. Stem Cells 2017;35:1316-27. [Crossref] [PubMed]
- Tapia N, Schöler HR. p53 connects tumorigenesis and reprogramming to pluripotency. J Exp Med 2010;207:2045-8. [Crossref] [PubMed]
- Hey CAB, Saltõkova KB, Bisgaard HC, et al. Comparison of two different culture conditions for derivation of early hiPSC. Cell Biol Int 2018;42:1467-73. [Crossref] [PubMed]
- Shiras A, Chettiar ST, Shepal V, et al. Spontaneous transformation of human adult nontumorigenic stem cells to cancer stem cells is driven by genomic instability in a human model of glioblastoma. Stem Cells 2007;25:1478-89. [Crossref] [PubMed]
- Doi D, Morizane A, Kikuchi T, et al. Prolonged maturation culture favors a reduction in the tumorigenicity and the dopaminergic function of human ESC-derived neural cells in a primate model of Parkinson's disease. Stem Cells 2012;30:935-45. [Crossref] [PubMed]
- Yamanaka S. Pluripotent Stem Cell-Based Cell Therapy-Promise and Challenges. Cell Stem Cell 2020;27:523-31. [Crossref] [PubMed]
- Zenke M. Human ES cell-derived dendritic cells: Meeting the challenge of immune rejection in allogeneic cell therapy. EBioMedicine 2020;62:103144. [Crossref] [PubMed]
- Deuse T, Hu X, Agbor-Enoh S, et al. De novo mutations in mitochondrial DNA of iPSCs produce immunogenic neoepitopes in mice and humans. Nat Biotechnol 2019;37:1137-44. [Crossref] [PubMed]
- Joel MDM, Yuan J, Wang J, et al. MSC: immunoregulatory effects, roles on neutrophils and evolving clinical potentials. Am J Transl Res 2019;11:3890-904. [PubMed]
- Contreras-Lopez R, Elizondo-Vega R, Luque-Campos N, et al. The ATP synthase inhibition induces an AMPK-dependent glycolytic switch of mesenchymal stem cells that enhances their immunotherapeutic potential. Theranostics 2021;11:445-60. [Crossref] [PubMed]
- Cheng RJ, Xiong AJ, Li YH, et al. Mesenchymal Stem Cells: Allogeneic MSC May Be Immunosuppressive but Autologous MSC Are Dysfunctional in Lupus Patients. Front Cell Dev Biol 2019;7:285. [Crossref] [PubMed]
- Pezzanite LM, Fortier LA, Antczak DF, et al. Equine allogeneic bone marrow-derived mesenchymal stromal cells elicit antibody responses in vivo. Stem Cell Res Ther 2015;6:54. [Crossref] [PubMed]
- Isakova IA, Lanclos C, Bruhn J, et al. Allo-reactivity of mesenchymal stem cells in rhesus macaques is dose and haplotype dependent and limits durable cell engraftment in vivo. PLoS One 2014;9:e87238. [Crossref] [PubMed]
- Ma T, Wang X, Jiao Y, et al. Interleukin 17 (IL-17)-Induced Mesenchymal Stem Cells Prolong the Survival of Allogeneic Skin Grafts. Ann Transplant 2018;23:615-21. [Crossref] [PubMed]
- Hu C, Li L. The immunoregulation of mesenchymal stem cells plays a critical role in improving the prognosis of liver transplantation. J Transl Med 2019;17:412. [Crossref] [PubMed]
- Deuse T, Hu X, Agbor-Enoh S, et al. The SIRPα-CD47 immune checkpoint in NK cells. J Exp Med 2021;218:e20200839. [Crossref] [PubMed]
- Xu H, Wang B, Ono M, et al. Targeted Disruption of HLA Genes via CRISPR-Cas9 Generates iPSCs with Enhanced Immune Compatibility. Cell Stem Cell 2019;24:566-578.e7. [Crossref] [PubMed]
- Kim A, Lee KG, Kwon Y, et al. Off-the-Shelf, Immune-Compatible Human Embryonic Stem Cells Generated Via CRISPR-Mediated Genome Editing. Stem Cell Rev Rep 2021;17:1053-67. [Crossref] [PubMed]
Cite this article as: Brianna, Ling APK, Wong YP. Applying stem cell therapy in intractable diseases: a narrative review of decades of progress and challenges. Stem Cell Investig 2022;9:4.



