Chromatin silencing maintains the identity of intestinal stem cells
The epithelial sheet of the small intestine harbors villi structures in the lumen and invaginations to form the crypts of Lieberkühn (1). Intestinal stem cells (ISCs) at the bottom of the crypts give rise to Paneth cells and transit-amplifying cells that are capable of differentiating into different types of cells that are components of the small intestine (1). Hierarchies of stem cell models suggest that there are 4 to 6 stem cells per crypt, which decide the ultimate pattern of the entire crypt (2). Two models were originally established for ISCs: the +4 label retaining cell (LRC) model and the crypt base columnar cell (CBC) or Lgr5+ cell model (2,3). Wnt signaling is known to be essential for Lgr5+ ISCs, and the bottom of the crypt is thought to be a stem cell niche in which Wnt signaling crosstalks with other signaling pathways in regulating self-renewal of ISCs as well as their differentiation, proliferation and migration (4).
At the +4 position of the crypt, an ISC marker, B lymphoma Mo-MLV insertion region 1 (Bmi1), is predominantly expressed (5). Bmi1 belongs to the Polycomb group (PcG) gene family, which functions in gene silencing through chromatin modifications. Through lineage analysis of repopulation kinetics of the Bmi1+ lineage, Sangiorgi et al. demonstrated that Bmi1-expressing stem cells are distributed along the length of the small intestine and suggested that mice use more than one adult stem cell subpopulation to maintain organ homeostasis (5). However, the precise role of PcG proteins in maintaining ISCs was not defined.
Recent study by Chiacchiera et al. demonstrated that the Polycomb repressive complex PRC1 plays a master role in maintaining homeostasis of the intestinal epithelium (6) (Figure 1). By coupling a constitutive knockout (KO) allele for Ring1a and a 4-hydroxytamoxifen (OHT)-inducible Cre-dependent KO allele for Ring1b in the mouse model, Chiacchiera et al. acutely inactivated PRC1 and observed a rapid loss of body weight coupled with a thinner intestine with impaired function in the mice. Loss of H2A monoubiquitination was due to loss of PRC1 activity, which played a direct role in controlling intestinal homeostasis in the adult mice (6).
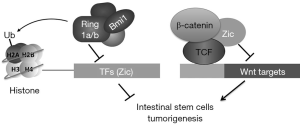
Using the ISC-specific Lgr5GFP-CreERT2 mouse model created by H. Clevers laboratory (7), Chiacchiera et al. investigated the role of PRC1 in H2A monoubiquitination and homeostasis in the intestine. They found that ISC-specific PRC1 ablation reduced ISC number and affected normal crypt architecture. Ring1a/b double KO mice showed degenerating crypts starting from 7 days post tamoxifen induction, and the number of these abnormal crypts increased after 15 days. All the degenerating crypts stained negative for ubiquitinated H2A. Fluorescence-activated cell sorting (FACS) analysis of these crypts showed a remarkable reduction of GFP+ ISCs, further demonstrating that loss of PRC1 leads to a robust reduction of the ISC pool in mouse crypts. Analysis in the cell spheroid culture also showed the essential role of PRC1 in preserving the homeostasis of the adult intestinal epithelium by maintaining ISC self-renewal independently from their niche (6).
Chiacchiera et al. further investigated whether PRC1 has a direct role in the maintenance of intestinal identity in the stem cells. By comparing both up- and down-regulated genes with the transcriptional profiles from different tissues, they found that PRC1 inactivation leads to loss of general intestinal lineage identity rather than to differentiation of ISCs. Using ChIP-seq, Chiacchiera et al. identified Ring1b- and H2Aubq-enriched genomic loci in both crypts and ISCs. RNA-seq analysis in the Ring1a/b double KO villi confirmed that PRC1 is required to maintain transcriptional repression in ISCs (6).
Analysis of gene ontology databases identified activation of DNA-binding transcription factors (TF) in Ring1a/b double KO ISCs, with the main function of PRC1 in suppressing the transcription of these genes in ISCs. Among the identified genes, Chiacchiera et al. demonstrated that simultaneous inhibition of Zic1 and Zic2 expression in HCT116 enhanced β-catenin/TCF transcriptional activity, while independent expression of either Zic1 or Zic2 induced intestinal organoid regression similarly to loss of PRC1 activity. Chiacchiera et al. further demonstrated that Zic1 or Zic2 expression inhibited β-catenin/TCF transcriptional activity via direct interaction with TCF7L2, thus affecting tissue homeostasis in the organoids. This model was further validated in mouse studies by combining β-catenin activation with loss of PRC1 activity in ISCs (6).
A previous study by Yu et al. suggested that Wnt signaling also regulates Bmi1 expression in normal intestine and colon cancer (8). Wnt signaling enhances c-Myc expression and c-Myc directly binds to Bmi1 promoter and activates its transcription. A Wnt repressor protein, KLF4, inhibited the expression of PRC proteins including Bmi1 and Ring1B and reduced histone monoubiquitination (8). Bmi1 is overexpressed in human colon cancers, and knocking down Bmi1 by shRNAs inhibited colon cancer xenografts in nude mice (8). The study by Chiacchiera et al. further explained the mechanism of how Bmi1 and other PRC proteins regulate adult stem cells in the intestine (Figure 1).
The above studies suggest that the PRC1 complex is a valuable drug target for colon cancer treatment. In fact, Kreso et al. reported that treatment of primary colorectal cancer xenografts with a small-molecular Bmi1 inhibitor led to reduced levels of histone monoubiquitination and loss of cancer initiating cells (9). This Bmi1 inhibitor represses the expression of Bmi1; it will be interesting to develop other small-molecular inhibitors targeting PRC1 activity in terms of histone ubiquitination.
Study by Chiacchiera et al. demonstrated that chromatin silencing could repress the expression of transcription factors that inhibit Wnt signaling and thus sustain the activity of Wnt signaling in the ISCs. The intestine stem cell is a fantastic research area; there are still many avenues to explore. For example, what are the roles of other transcription factors repressed by PRC1 and what are the different roles of PRC1 in Lgr5+ ISCs and +4 ISCs? Zic has been reported as both positive and negative regulator of Wnt (10); further investigation of the mechanism and function of Zic in the intestine may lead to other unexpected findings.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Crosnier C, Stamataki D, Lewis J. Organizing cell renewal in the intestine: stem cells, signals and combinatorial control. Nat Rev Genet 2006;7:349-59. [Crossref] [PubMed]
- van der Flier LG, Clevers H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu Rev Physiol 2009;71:241-60. [Crossref] [PubMed]
- Scoville DH, Sato T, He XC, et al. Current view: intestinal stem cells and signaling. Gastroenterology 2008;134:849-64. [Crossref] [PubMed]
- Pardal R, Clarke MF, Morrison SJ. Applying the principles of stem-cell biology to cancer. Nat Rev Cancer 2003;3:895-902. [Crossref] [PubMed]
- Sangiorgi E, Capecchi MR. Bmi1 is expressed in vivo in intestinal stem cells. Nat Genet 2008;40:915-20. [Crossref] [PubMed]
- Chiacchiera F, Rossi A, Jammula S, et al. Polycomb complex PRC1 preserves intestinal stem cell identity by sustaining Wnt/β-catenin transcriptional activity. Cell Stem Cell 2016;18:91-103. [Crossref] [PubMed]
- Barker N, van Es JH, Kuipers J, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 2007;449:1003-7. [Crossref] [PubMed]
- Yu T, Chen X, Zhang W, et al. Regulation of the potential marker for intestinal cells, Bmi1, by β-catenin and the zinc finger protein KLF4: implications for colon cancer. J Biol Chem. 2012;287:3760-8. [Crossref] [PubMed]
- Kreso A, van Galen P, Pedley NM, et al. Self-renewal as a therapeutic target in human colorectal cancer. Nat Med 2014;20:29-36. [Crossref] [PubMed]
- Frank CL, Liu F, Wijayatunge R, et al. Regulation of chromatin accessibility and Zic binding at enhancers in the developing cerebellum. Nat Neurosci 2015;18:647-56. [Crossref] [PubMed]
Cite this article as: Yu T, Liu C. Chromatin silencing maintains the identity of intestinal stem cells. Stem Cell Investig 2016;3:20.


