Successful treatment of ibrutinib-associated central nervous system hemorrhage with platelet transfusion support
Introduction
Traditional chemotherapy agents were the mainstay of therapy for patients with chronic lymphoid leukemia (CLL) and mantle cell lymphoma for many years. Recently, the approval of several new agents with targeted mechanisms of action has resulted in a paradigm shift in the treatment of these diseases. However after the relatively rapid approval of new drugs, the full toxicity profile may not be apparent until a broader population of patients is exposed in the post marketing setting.
Ibrutinib is a Bruton tyrosine kinase inhibitor (1,2) that is approved for the treatment of mantle cell lymphoma after at least one prior therapy (3), chronic lymphocytic leukemia with or without a 17p deletion (4-6) and Waldenstrom’s macroglobulinemia (7). The most common toxicities reported are cytopenias, diarrhea, fatigue, infection, musculoskeletal pain and bruising. Bleeding events occurred in all of the trials but were grade 1 or 2 in most patients. In the mantle cell lymphoma trial, bleeding occurred in 48% of patients (3), with 5% of patients having grade 3 or higher bleeding events including subdural hematoma, gastrointestinal bleeding, or hematuria. In the phase II CLL trial bruising was reported in 54% of patients (4), and was grade 3/4 in 2% of cases. In the phase III CLL trial, four patients treated with ibrutinib had grade 3 hemorrhage and one had grade 4 hemorrhage (5).
We recently treated two patients who developed life threatening central nervous system (CNS) bleeding while on ibrutinib. Both patients had a platelet count that was above that which would be expected to result in spontaneous bleeding. Both patients had cessation of bleeding after administration of platelet transfusions.
Case presentation
Patient 1
The patient is a 66 years old woman who was diagnosed with CLL in 2005 after routine blood work demonstrated lymphocytosis. In 2012 she received 4 cycles of fludarabine, cytoxan, rituxan (FCR) for the treatment of progressive lymphocytosis and lymphadenopathy. She was again well until February 2015 when she developed progressive lymphocytosis, lymphadenopathy and splenomegaly. The patient was treated with ibrutinib 420 mg orally daily. She was not taking any other medications. She tolerated therapy well until November 2015 when she awoke suddenly with intractable vomiting and confusion. The patient was transported to a local hospital where, upon arrival, she was unresponsive with a Glasgow coma score of 8. She was intubated for airway protection and sedated. A CT scan showed acute intraventricular hemorrhage with subarachnoid blood. The patient was transferred to our hospital for further therapy.
Upon arrival at our hospital there were no extraocular movements and the pupils were 2 mm. The patient localized to noxious stimuli. Sedation was held for neurological evaluation. There was no lymphadenopathy or hepatosplenomegaly palpable. Laboratories showed a white blood count (WBC) of 165,000/mm3 with 6% neutrophils, 85% lymphocytes, 1% monocytes, and 8% atypical lymphocytes. The hemoglobin was 13.8 g/dL, and the platelet count was 137,000/mm3. Peripheral blood flow cytometry confirmed the diagnosis of CLL with 87% of cells co-expressing CD5, CD19, CD20, CD22, and CD23 (heterogeneous). Fluorescence in situ hybridization (FISH) subsequently demonstrated abnormalities of 6q- and 13q-. The IGH gene variable region was unmutated. Results of platelet aggregation studies and other coagulation tests which were performed are summarized in Table 1. The only abnormality detected was a mild decrease in reaction to collagen (59%) on platelet aggregation studies.
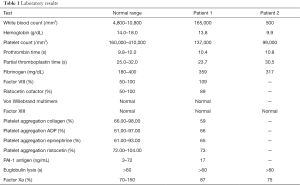
Full table
A non-contrast CT scan of the head demonstrated extensive intraventricular hemorrhage within the occipital horns of the lateral ventricles, third ventricle, and fourth ventricle. There was also a small amount of acute subarachnoid hemorrhage within the sylvian fissures. There was hydrocephalus with dilation of the ventricular system and there was partial effacement of the cerebral hemispheric sulci. A CT angiogram of the head and neck demonstrated no evidence of intracranial aneurysm nor arteriovenous malformation. An MRI demonstrated extensive gradient hypointensity within the ventricular system consistent with acute intraventricular hemorrhage, as well as MRI findings consistent with subarachnoid hemorrhage. Additionally there was moderate atrophy and moderate chronic small vessel ischemic changes.
A ventriculostomy was placed. The patient was given single donor platelet transfusions every 6 h for a total of 8 transfusions. The patient improved rapidly and was discharged to a rehabilitation unit 11 days after admission. At the time of discharge the patient was awake and alert and fully ambulatory. She had residual deficits in the extraocular muscle movements of the right eye and a mildly altered affect at the time of transfer. At a 3 months follow-up visit her modified Rankin score was 0, she had no deficits and her speech was normal.
Patient 2
The patient was an 80-year-old man with history of chronic obstructive pulmonary disease, atrial fibrillation on warfarin, glaucoma, and basal cell carcinoma. He initially presented in January 2012 with cervical lymphadenopathy. A fine needle aspiration performed at an outside institution showed small lymphocytic leukemia. Initially a watch and wait approach was chosen. In July of 2015 the patient presented with progressive lymphadenopathy and a chest wall mass. Core biopsy of the mass demonstrated a diffuse lymphoid infiltrate. The lymphocytes were medium to large in size with fine chromatin. Immunohistochemistry showed that the cells were positive for CD20, PAX-5, CD5, BCL-1, SOX-11, BCL-2, and negative for MUM-1, CD23, BCL-6, CD10. The Ki67 was 90% and FISH demonstrated the CCND1/IGH rearrangement. The findings were consistent with blastoid variant mantle cell lymphoma.
Due to the patient’s age and comorbities he received a less intensive regimen than is typical for this disease. He received 3 cycles of bendamustine and rituxan. Although he initially demonstrated a significant reduction in the size of the mass and lymph nodes, he had clinical progression after the third cycle of therapy. Concurrently the patient developed a right third cranial nerve palsy. Lumbar puncture confirmed lymphomatous meningitis.
The patient was transferred to our care. After discontinuation of his anticoagulation an Ommaya catheter was placed. He received intrathecal methotrexate and was discharged to receive treatment closer to home. The patient was treated with ASA 81 mg daily rather than warfarin for his high risk cardiac disease. Two weeks later the patient began ibrutinib 420 mg orally daily concurrently with intrathecal methotrexate given on a weekly schedule. Three weeks later the patient returned with severe fatigue. On physical exam the patient was lethargic and febrile. The complete blood count showed a WBC of 500/mm3 with 88% neutrophils and 12% lymphocytes. The hemoglobin was 9.9 g/dL and the platelet count was 99,000/mm3. The LDH was 1,392 U/L, the creatinine was 1.63 mg/dL and the uric acid was 22.3 units. Coagulation studies were unremarkable and are listed in Table 1.
CT scan of the brain demonstrated bilateral holohemispheric subdural collections up to 1 cm in maximal thickness, which was hyperdense compared to CSF. The left-sided collection had a higher density component compatible with acute on subacute subdural hemorrhage. The right-sided collection was favored to represent a subacute subdural hemorrhage. There was no shift of midline structures or hydrocephalus.
The patient was treated with intravenous antibiotics, filgrastim, steroids and rasburicase. Single donor platelets were given every 6 h for a total of 17 transfusions. A follow up CT showed the expected evolution of blood products but no further bleeding. The patient subsequently developed pneumonia and respiratory failure and, after palliative therapy was initiated, he expired.
Discussion
Ibrutinib is a targeted agent that is highly active against a range of B-cell malignancies. Although bleeding events were noted in the clinical trials, few details of the patients’ coagulation status, treatment and outcome were reported.
In vitro studies have recently demonstrated that platelet aggregation abnormalities occur after exposure to ibrutinib. Rigg reported that irreversible inhibition of BTK with two ibrutinib analogs significantly decreased glycoprotein VI-mediated platelet activation, spreading, and aggregation in vitro (8). Likewise, Bye demonstrated that platelets treated with ibrutinib adhered to collagen under arterial shear but formed unstable thrombi and that integrin αIIbβ3 outside-in signaling was also effected in addition to glycoprotein VI signaling (9). The authors concluded that ibrutinib causes glycoprotein VI and integrin αIIbβ3 platelet signaling deficiencies that result in formation of unstable thrombi and that combining ibrutinib with P2Y12 antagonists may have a detrimental effect on hemostasis.
Lipsky et al. prospectively assessed platelet function and coagulation factors in 85 patients treated on a trial of ibrutinib for chronic lymphocytic leukemia (10). After a median follow-up of 24 months the authors recorded grade 1 or 2 bleeding events in 55% of the patients. No grade 3/4 events occurred. The median time to bleeding was 49 days and the risk of an event plateaued by 6 months. In this trial, von Willebrand factor and factor VIII levels were often high at baseline and then normalized on treatment. Collagen and adenosine diphosphate induced platelet aggregation was decreased in all patients with chronic lymphocytic leukemia, whether on ibrutinib or not. Compared to untreated chronic lymphocytic leukemia patients, response to collagen showed a mild further decrement on ibrutinib, while response to adenosine diphosphate improved. The authors concluded that both disease and treatment-related factors influenced the risk of bleeding. In a smaller clinical study, Levade et al. demonstrated that ibrutinib selectively inhibited platelet signaling and functions downstream of the collagen receptor glycoprotein VI and strongly affected firm platelet adhesion on von Willebrand factor under arterial flow (11). The addition of 50% untreated platelets was sufficient to efficiently reverse the effects of ibrutinib, and platelet functions recovered after treatment interruption as physiological platelet renewal occurred. Kamel et al. also noted that 23 patients receiving ibrutinib all had reductions in collagen-mediated platelet aggregation, and that there was a significant association between the degree of inhibition and the occurrence of clinical bleeding or bruising (12).
Our first patient had no major risk factors for CNS bleeding and developed life threatening hemorrhage without any warning. The only abnormality found was a very mild reduction in platelet aggregation in response to collagen. Our second patient multiple risk factors for hemorrhage. He had a recent neurosurgical procedure as well as lymphomatous meningitis. He also had a history of atrial fibrillation and was on low dose aspirin at the time of his hemorrhage. Due to the reports of platelet dysfunction we treated both of our patients with platelet transfusions, even though one had a normal platelet count and the other had only a mild reduction in platelet count. The first patient had a dramatic improvement in her clinical condition after the infusions. The second patient had resolution of bleeding, but opted for comfort care after he developed pneumonia and he subsequently expired from causes unrelated to the hemorrhage.
It is impossible to know if the resolution of bleeding was directly related to the platelet transfusions administered or was just due to clearance of ibrutinib and production of new platelets by the patient. However, in view of the favorable bleeding outcomes, we propose that this is a reasonable approach to the patient who has life threatening bleeding while on ibrutinib.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: The written informed consent was obtained from the patient who is alive for publication of this Case report and any accompanying images. We failed to obtain consent from family members or caregivers of the deceased patient.
References
- Honigberg LA, Smith AM, Sirisawad M, et al. The Bruton tyrosine kinase inhibitor PCI-32765 blocks B-cell activation and is efficacious in models of autoimmune disease and B-cell malignancy. Proc Natl Acad Sci U S A 2010;107:13075-80. [Crossref] [PubMed]
- Herman SE, Gordon AL, Hertlein E, et al. Bruton tyrosine kinase represents a promising therapeutic target for treatment of chronic lymphocytic leukemia and is effectively targeted by PCI-32765. Blood 2011;117:6287-96. [Crossref] [PubMed]
- Wang ML, Rule S, Martin P, et al. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N Engl J Med 2013;369:507-16. [Crossref] [PubMed]
- Byrd JC, Furman RR, Coutre SE, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med 2013;369:32-42. [Crossref] [PubMed]
- Byrd JC, Brown JR, O’Brien S, et al. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med 2014;371:213-23. [Crossref] [PubMed]
- Burger JA, Tedeschi A, Barr PM, et al. Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N Engl J Med 2015;373:2425-37. [Crossref] [PubMed]
- Treon SP, Tripsas CK, Meid K, et al. Ibrutinib in previously treated Waldenström's macroglobulinemia. N Engl J Med 2015;372:1430-40. [Crossref] [PubMed]
- Rigg RA, Aslan JE, Healy LD, et al. Oral administration of Bruton's tyrosine kinase inhibitors impairs GPVI-mediated platelet function. Am J Physiol Cell Physiol 2016;310:C373-80. [Crossref] [PubMed]
- Bye AP, Unsworth AJ, Vaiyapuri S, et al. Ibrutinib inhibits platelet integrin αIIbβ3 outside-in signaling and thrombus stability but not adhesion to collagen. Arterioscler Thromb Vasc Biol 2015;35:2326-35. [Crossref] [PubMed]
- Lipsky AH, Farooqui MZ, Tian X, et al. Incidence and risk factors of bleeding-related adverse events in patients with chronic lymphocytic leukemia treated with ibrutinib. Haematologica 2015;100:1571-8. [Crossref] [PubMed]
- Levade M, David E, Garcia C, et al. Ibrutinib treatment affects collagen and von Willebrand factor-dependent platelet functions. Blood 2014;124:3991-5. [Crossref] [PubMed]
- Kamel S, Horton L, Ysebaert L, et al. Ibrutinib inhibits collagen-mediated but not ADP-mediated platelet aggregation. Leukemia 2015;29:783-7. [Crossref] [PubMed]
Cite this article as: Seiter K, Stiefel MF, Barrientos J, Shaikh A, Ahmed N, Baskind P, Liu D. Successful treatment of ibrutinib-associated central nervous system hemorrhage with platelet transfusion support. Stem Cell Investig 2016;3:27.


