Pancreatic DCLK1 marks quiescent but oncogenic progenitors: a possible link to neuroendocrine tumors
Doublecortin-like kinase 1 (DCLK1) is a microtubule-associated protein that plays key roles in the regulation of neural cell differentiation, migration, and apoptosis during embryonic development (1,2). Accumulating evidence suggests that DCLK1 is a marker of intestinal and pancreatic stem cells and cancer stem cells; thus, it is attracting attention from both gastroenterologists and oncologists (3-6). The function of DCLK1 is not fully understood; however, at minimum, we know that this kinase induces epithelial-mesenchymal transition (EMT), which supports the stemness of normal and neoplastic stem cells. Regarding mechanism of the DCLK1-induced EMT, involvement of specific microRNA-dependent upregulation of c-MYC, KRAS, and Notch1 expressions was demonstrated in pancreatic and colon cancer cells (7,8). Furthermore, a recent study has strengthened the evidence by shedding light on upregulation the EMT regulator SLUG by DCLK1 that resulted in increased cell migration ability (9).
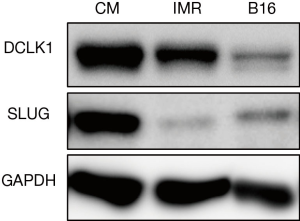
The location of and markers for adult pancreatic progenitor cells have long been debated. Westphalen et al. have recently demonstrated in their elegant lineage-tracing study that DCLK1 labeled a rare population of long-lived, quiescent pancreatic progenitor cells that were necessary for pancreatic regeneration following injury and chronic inflammation (6). Of interest, these quiescent DCLK1+ cells did not contribute to carcinogenesis even in the setting of KRAS mutation; however, experimental pancreatitis converted the KRAS-mutated DCLK1+ cells into potent cancer-initiating cells. A similar oncogenic role of DCLK1, activated by tissue injury, was also exhibited in colorectal cancer (10). Despite such emerging recognized roles of DCLK1 in the stem cell milieu, why the neural stem/progenitor marker DCLK1 universally labels stem/progenitors of non-neural organs, including the pancreas and colorectum, has not been eagerly discussed. Because DCLK1 was clearly expressed in both neuroblastoma and melanoma cells (Figure 1) (11), which are derived from neural crest cells (NCCs) (12,13), it is speculated that DCLK1+ stem/progenitors in various organs originate from a neural-crest–derived cell population. Indeed, these tumors are clinically aggressive and metastatic, exhibiting increased migratory potential similar to NCCs. Neuroendocrine tumors (NETs), which robustly express DCLK1 (9), are also known to be highly metastatic. Thus, DCLK1 positivity may be the key to understanding the tumor-cell behaviors of highly metastatic tumors, including NETs, neuroblastomas, and melanomas. SLUG, a crucial EMT regulator downstream of DCLK1, is expressed in all three tumor cell types described above (Figure 1). Because SLUG is also an inevitable driver of EMT in NCCs (14), it is suggested that the high metastatic potential of DCLK1+ tumor cells may be attributable to their common cell origin, i.e., the neural crest. Thus, an examination on whether DCLK1 is expressed in NCCs and regulates their migration ability is required. Additionally, a lineage-tracing study focusing on NCCs would address the neural crest’s involvement in the development and maintenance of quiescent and long-lived stem/progenitor cells in adult organs and cancers.
Acknowledgements
Funding: This work was supported by in part by the Japan Society for the Promotion of Science (JSPS) KAKENHI (No. 15K19355).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Shu T, Tseng HC, Sapir T, et al. Doublecortin-like kinase controls neurogenesis by regulating mitotic spindles and M phase progression. Neuron 2006;49:25-39. [Crossref] [PubMed]
- Koizumi H, Higginbotham H, Poon T, et al. Doublecortin maintains bipolar shape and nuclear translocation during migration in the adult forebrain. Nat Neurosci 2006;9:779-86. [Crossref] [PubMed]
- May R, Sureban SM, Lightfoot SA, et al. Identification of a novel putative pancreatic stem/progenitor cell marker DCAMKL-1 in normal mouse pancreas. Am J Physiol Gastrointest Liver Physiol 2010;299:G303-10. [Crossref] [PubMed]
- Nakanishi Y, Seno H, Fukuoka A, et al. Dclk1 distinguishes between tumor and normal stem cells in the intestine. Nat Genet 2013;45:98-103. [Crossref] [PubMed]
- Bailey JM, Alsina J, Rasheed ZA, et al. DCLK1 marks a morphologically distinct subpopulation of cells with stem cell properties in preinvasive pancreatic cancer. Gastroenterology 2014;146:245-56. [Crossref] [PubMed]
- Westphalen CB, Takemoto Y, Tanaka T, et al. Dclk1 defines quiescent pancreatic progenitors that promote injury-induced regeneration and tumorigenesis. Cell Stem Cell 2016;18:441-55. [Crossref] [PubMed]
- Sureban SM, May R, Lightfoot SA, et al. DCAMKL-1 regulates epithelial-mesenchymal transition in human pancreatic cells through a miR-200a-dependent mechanism. Cancer Res 2011;71:2328-38. [Crossref] [PubMed]
- Chandrakesan P, Weygant N, May R, et al. DCLK1 facilitates intestinal tumor growth via enhancing pluripotency and epithelial mesenchymal transition. Oncotarget 2014;5:9269-80. [Crossref] [PubMed]
- Ikezono Y, Koga H, Akiba J, et al. DCLK1 promotes tumor growth and invasion through Slug-mediated epithelial-mesenchymal transition in pancreatic neuroendocrine tumors. Proceedings of the 107th Annual Meeting of the American Association for Cancer Research; 2016 Apr 16-20; New Orleans, LA. Philadelphia (PA): AACR; 2016:Abstract nr 1728.
- Westphalen CB, Asfaha S, Hayakawa Y, et al. Long-lived intestinal tuft cells serve as colon cancer-initiating cells. J Clin Invest 2014;124:1283-95. [Crossref] [PubMed]
- Ikezono YU, Koga H, Abe M, et al. High expression of the putative cancer stem cell marker, DCLK1, in rectal neuroendocrine tumors. Oncol Lett 2015;10:2015-20. [PubMed]
- Marshall GM, Carter DR, Cheung BB, et al. The prenatal origins of cancer. Nat Rev Cancer. 2014;14:277-89. [Crossref] [PubMed]
- Shakhova O. Neural crest stem cells in melanoma development. Curr Opin Oncol 2014;26:215-21. [Crossref] [PubMed]
- Tien CL, Jones A, Wang H, et al. Snail2/Slug cooperates with Polycomb repressive complex 2 (PRC2) to regulate neural crest development. Development 2015;142:722-31. [Crossref] [PubMed]
Cite this article as: Koga H, Ikezono Y, Torimura T. Pancreatic DCLK1 marks quiescent but oncogenic progenitors: a possible link to neuroendocrine tumors. Stem Cell Investig 2016;3:37.


