Wnt signalling meets epigenetics
The Wnt signaling pathway is required for the proper function of hematopoietic stem cells (HSCs), but its level is subject to strict regulatory control. Too much Wnt signaling leads to exhaustion of HSCs due to enhanced differentiation and loss of self-renewal. Recent work from several laboratories has indicated the complex mechanisms that are involved in normal and malignant regulation of hematopoiesis by Wnt signals. Very recently, epigenetic changes that modify the activation of genes, but not their DNA sequence, have been implicated as well. That is, specific deletion of the epigenetic regulator of histone methylation, Sirtuin 6 in blood cells, increases Wnt signaling. Here, I will briefly discuss these novel insights into Wnt signaling and HSCs.
Introduction
The stem cells of the blood have set the paradigm for all stem cell systems, i.e., a rare cell being on top of the hierarchy of differentiating daughter cells from different lineages and having the ability to self-renew (1). Hematopoiesis, the process of blood formation, has been the experimental system of choice for the phenotypic, molecular and functional definition of stem cells. An overwhelming number of studies have dealt with signaling pathways governing HSC fate (either self-renewal, proliferation, differentiation, apoptosis, or quiescence), including the evolutionary conserved Wnt pathway [for instance reviewed in (2,3)]. Other important pathways such as Notch, hedgehog, SMAD, FGF and receptor tyrosine kinase also regulate HSC biology [see for instance (4)]. However, in contrast to other stem cell systems, where Wnt signaling is the major driving force for self-renewal (5), the role of Wnt signaling in blood stem cells has been somewhat controversial [recently reviewed in (6)]. This probably has to do with the relatively low levels of Wnt signaling required for normal HSC function compared to for instance intestinal stem cells and with the fact that much higher levels of Wnt signaling invariably lead to stem cell exhaustion in blood (7,8).
Wnt signaling
The Wnt signaling cascade is often discerned into the canonical or Wnt/β-catenin pathway and the non-canonical pathways that do not use β-catenin as protein to transfer the cytoplasmic Wnt signal to a nuclear response (9-13). In the canonical pathway, cytoplasmic levels of β-catenin are kept very low through the action of a protein complex (the so-called destruction complex) that actively targets β-catenin for degradation. Activation of the canonical pathway by binding of a Wnt protein to a Frizzled receptor, leads to inactivation of the destruction complex allowing built-up of the dephosphorylated form of β-catenin and its migration to the nucleus. In the nucleus, β-catenin binds to members of the TCF/LEF transcription factor family, thereby converting them from transcriptional repressors into transcriptional activators. It should be noted that TCF/LEF factors also function as transcriptional repressors, in part through binding of Groucho-TLE corepressors (14). Also in hematopoietic cells the repressor function of these factors is important, as for instance shown in the thymus where loss of Tcf1 predisposes to the development of leukemia (15,16).
Non-canonical Wnt signaling uses Wnt/Fz ligand/receptors yet does not signal via β-catenin, but uses JNK kinases, intracellular free calcium or entirely other receptors for instance Ryk or Ror (13). Biologically, they can inhibit the canonical pathway or are involved in cell polarity or cell motility. Importantly, non-canonical Wnt signaling has been proposed to regulate quiescence of HSCs (11,17).
Epigenetics and Sirtuins
Besides through various signaling pathways, HSC are also subject to epigenetic control. Epigenetic changes modify the activation of genes, but not their DNA sequence. Common epigenetic changes are methylation of DNA (e.g., cytosine methylation in so-called CpG islands) or modifications of histone proteins (e.g., lysine acetylation, lysine and arginine methylation, serine and threonine phosphorylation, and lysine ubiquitination and sumoylation) (18). Especially acetylation of histone proteins has been highly studied as it directly affects the activity of the nucleosome-associated genes. Histone acetyltransferase enzymes (HATs) and histone deacetylases (HDACs) regulate the acetylation status of histone proteins, in particular H3 and H4. The Sirtuin family of proteins also have deacetylase activity and are thought to play key roles in metabolism, immunity, ageing and apoptosis [reviewed in e.g., (19)]. In mammals there are seven Sirtuins (SIRT1-7). Interestingly, the HDAC activity of sirtuins is dependent on NAD(P) and therefore directly linked to the energy metabolism of cells. Indeed the function of some Sirtuins (Sirt3, Sirt7) is intimately linked to proper mitochondrial function. Sirt3 (20) and Sirt7 deficiency (21) both affect HSC self-renewal by influencing the function of mitochondria and the unfolded protein response, thereby mimicking stem cell ageing. Sirt6 deficient mice also have an aging phenotype, but transplantation of Sirt6−/− bone marrow cells showed surprisingly little effect (22). This is in sharp contrast to an elaborate recent study in which Sirt6 was conditionally deleted in the hematopoietic system (23).
Targeted mutation of Sit6 in HSC
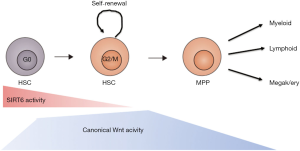
The function of Sirt6 in HSC was studied by Wang et al. in detail using a floxed version rather than straight knockout of the gene (23). To their credit, these investigators not only used the sometimes problematic Mx-Cre system (which requires poly I-C injections thereby mimicking an inflammatory response which effects many cells, such as intestinal epithelium in addition to blood cells) but also ERT2-Cre and Vav-cre. They observed an increase in phenotypic HSCs, yet these were hyper activated, residing much more in G1/S than wild type HSC, showed increased proliferative capacity leading to eventual HSC exhaustion. Indeed in serial transplantation experiments these HSCs performed much less well than non floxed Sirt6+/+ HSC (23). Sirt6 normally binds to TCF factors and deacetylates lysine residue 56 (K56) in H3 thereby suppressing canonical Wnt target genes (such as Tcf7, Axin2, Ccnd1 and c-Myc). Concordingly, a Tcf-Lef Wnt reporter construct showed 5- to 6-fold higher Wnt signalling activity in phenotypically defined HSC (the so-called LSK population). This is in line with previous work by Luis et al. who showed that a 2.5-fold increase in Wnt signalling led to better hematopoietic reconstitution but a 5-fold (or more) increase in Wnt dosage did not give any reconstitution of recipient mice (24). Because Sirt6 has many pleiotropic effects authors also conducted a control experiment using a pharmacological inhibitor of Wnt signalling (ICG-001) to show that inhibition of Wnt signalling partially reversed the loss of repopulating capacity in Sirt6−/− HSCs, thereby further substantiating the notion that enhanced Wnt signalling in stem cells was causative for the observed effects. ICG-001 works somewhat indirect by competing with β-catenin for CBP (CREB binding protein) thereby inhibiting TCF/β-catenin mediated transcription (25). CBP or its homolog p300 act as transcriptional co-activators that are recruited by the TCF-catenin complex. ICG-001 inhibits CBP but not p300, so not all target genes are expected to be fully affected by this drug. Of interest, the Wnt target genes affected were similar to those recently found in gene expression profiling studies using floxed Apc alleles in HSCs (26). APC is a negative regulator of the pathway and when deleted in HSCs leads to upregulation of Wnt target genes such as Axin2, Tcf7 and CyclinD2 and subsequently to stem cell exhaustion. Famili et al. showed that this stem cell exhaustion is mainly caused by enhanced differentiation into both granulocytic and B lymphoid lineages (26). Taken together, Sirt6 functions to prevent hyper activation of Wnt signalling under quiescent conditions, and is less active under inflammatory and stressed situations when canonical Wnt signals activate HSCs to produce more blood cells (see Figure 1). Sirtuins together with other factors ensure that under the specific conditions, the “juts-right” levels of Wnt signalling for that condition are activated.
Non-canonical Wnt signalling
While the work on Sirt6 discussed above indicates that it functions to dampen Wnt signaling to prevent hyper proliferation of HSCs, other related work has shown that non-canonical Wnt signaling is required to maintain HSC quiescence. Frizzled8, together with Flamingo, is a non-canonical Wnt receptor that responds to non-canonical Wnts such as Wnt5a. Under stressed conditions, canonical Wnt signaling is enhanced to activate HSCs and induce self-renewal and differentiation (17). Indeed, non-canonical Wnt5a signaling is increased in short-term HSC repopulation by maintaining HSCs in a quiescent G(0) state (27). It would be interesting to explore if canonical and non-canonical Wnt signalling are differentially affected in Sirt6 deficient HSCs. Such differential effects of canonical (exemplified by Wnt3a) and non-canonical (Wnt5a mediated) signalling was recently shown in HSCs, where Wnt3a led to higher lymphoid output whereas Wnt5a led to higher myeloid output in vivo (28).
Future directions
The role of epigenetic changes with regards to Wnt signalling has been highlighted in haematological malignancies where methylation of key Wnt genes has been demonstrated (29-32). It would be of great interest to see if Sirtuins play a role in human haematological malignancies. Also, given the changes in Sirt6 expression in the thymus (Staal, unpublished) and the importance of Wnt signalling for thymic development (33,34), a role for Sirt6 in T cell development can be predicted. Such a role can be directly addressed using Lck-Cre or CD4-cre mice crossed to the floxed mice used by Wang et al. (23).
Wnt signalling is explored as a way to expand HSC ex vivo. However this is not an easy task by using Wnt alone, hence other signals such as PI3K/Akt signalling (35), as well as expression of Bcl2 (36) can provide such signals. Inhibition of the mTOR pathway using rapamycin, together with pharmacological activation of the Wnt pathway, has also been proposed (37). Based on the new insights discussed here, it would be worthwhile to consider controlled epigenetic changes in to such expansion protocols as well.
Acknowledgements
Funding: This work was supported in part by a TOP grant from The Netherlands Organization for Health Research and Development (ZonMw Project 40-00812-98-09050) to FJ Staal.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Staal FJ, Baum C, Cowan C, et al. Stem cell self-renewal: lessons from bone marrow, gut and iPS toward clinical applications. Leukemia 2011;25:1095-102. [Crossref] [PubMed]
- Malhotra S, Kincade PW. Wnt-related molecules and signaling pathway equilibrium in hematopoiesis. Cell Stem Cell 2009;4:27-36. [Crossref] [PubMed]
- Staal FJ, Luis TC, Tiemessen MM. WNT signalling in the immune system: WNT is spreading its wings. Nat Rev Immunol 2008;8:581-93. [Crossref] [PubMed]
- Blank U, Karlsson G, Karlsson S. Signaling pathways governing stem-cell fate. Blood 2008;111:492-503. [Crossref] [PubMed]
- Clevers H. Wnt/beta-catenin signaling in development and disease. Cell 2006;127:469-80. [Crossref] [PubMed]
- Luis TC, Ichii M, Brugman MH, et al. Wnt signaling strength regulates normal hematopoiesis and its deregulation is involved in leukemia development. Leukemia 2012;26:414-21. [Crossref] [PubMed]
- Kirstetter P, Anderson K, Porse BT, et al. Activation of the canonical Wnt pathway leads to loss of hematopoietic stem cell repopulation and multilineage differentiation block. Nat Immunol 2006;7:1048-56. [Crossref] [PubMed]
- Scheller M, Huelsken J, Rosenbauer F, et al. Hematopoietic stem cell and multilineage defects generated by constitutive beta-catenin activation. Nat Immunol 2006;7:1037-47. [Crossref] [PubMed]
- Qiang YW, Rudikoff S. Wnt signaling in B and T lymphocytes. Front Biosci 2004;9:1000-10. [Crossref] [PubMed]
- Staal FJ, Chhatta A, Mikkers H. Caught in a Wnt storm: Complexities of Wnt signaling in hematopoiesis. Exp Hematol 2016;44:451-7. [Crossref] [PubMed]
- Suda T, Arai F. Wnt signaling in the niche. Cell 2008;132:729-30. [Crossref] [PubMed]
- Veeman MT, Axelrod JD, Moon RT. A second canon. Functions and mechanisms of beta-catenin-independent Wnt signaling. Dev Cell 2003;5:367-77. [Crossref] [PubMed]
- Semenov MV, Habas R, Macdonald BT, et al. SnapShot: noncanonical wnt signaling pathways. Cell 2007;131:1378. [Crossref] [PubMed]
- Roose J, Molenaar M, Peterson J, et al. The Xenopus Wnt effector XTcf-3 interacts with Groucho-related transcriptional repressors. Nature 1998;395:608-12. [Crossref] [PubMed]
- Yu S, Zhou X, Steinke FC, et al. The TCF-1 and LEF-1 transcription factors have cooperative and opposing roles in T cell development and malignancy. Immunity 2012;37:813-26. [Crossref] [PubMed]
- Tiemessen MM, Baert MR, Schonewille T, et al. The nuclear effector of Wnt-signaling, Tcf1, functions as a T-cell-specific tumor suppressor for development of lymphomas. PLoS Biol 2012;10:e1001430. [Crossref] [PubMed]
- Sugimura R, He XC, Venkatraman A, et al. Noncanonical Wnt signaling maintains hematopoietic stem cells in the niche. Cell 2012;150:351-65. [Crossref] [PubMed]
- Paluch BE, Naqash AR, Brumberger Z, et al. Epigenetics: a primer for clinicians. Blood Rev 2016;30:285-95. [Crossref] [PubMed]
- Chalkiadaki A, Guarente L. The multifaceted functions of sirtuins in cancer. Nat Rev Cancer 2015;15:608-24. [Crossref] [PubMed]
- Brown K, Xie S, Qiu X, et al. SIRT3 reverses aging-associated degeneration. Cell Rep 2013;3:319-27. [Crossref] [PubMed]
- Mohrin M, Shin J, Liu Y, et al. Stem cell aging. A mitochondrial UPR-mediated metabolic checkpoint regulates hematopoietic stem cell aging. Science 2015;347:1374-7. [Crossref] [PubMed]
- Mostoslavsky R, Chua KF, Lombard DB, et al. Genomic instability and aging-like phenotype in the absence of mammalian SIRT6. Cell 2006;124:315-29. [Crossref] [PubMed]
- Wang H, Diao D, Shi Z, et al. SIRT6 controls hematopoietic stem cell homeostasis through epigenetic regulation of Wnt signaling. Cell Stem Cell 2016;18:495-507. [Crossref] [PubMed]
- Luis TC, Naber BA, Roozen PP, et al. Canonical wnt signaling regulates hematopoiesis in a dosage-dependent fashion. Cell Stem Cell 2011;9:345-56. [Crossref] [PubMed]
- Emami KH, Nguyen C, Ma H, et al. A small molecule inhibitor of beta-catenin/CREB-binding protein transcription. Proc Natl Acad Sci U S A 2004;101:12682-7. [Crossref] [PubMed]
- Famili F, Brugman MH, Taskesen E, et al. High levels of canonical wnt signaling lead to loss of stemness and increased differentiation in hematopoietic stem cells. Stem Cell Reports 2016;6:652-9. [Crossref] [PubMed]
- Nemeth MJ, Topol L, Anderson SM, et al. Wnt5a inhibits canonical Wnt signaling in hematopoietic stem cells and enhances repopulation. Proc Natl Acad Sci U S A 2007;104:15436-41. [Crossref] [PubMed]
- Famili F, Naber BA, Vloemans S, et al. Discrete roles of canonical and non-canonical Wnt signaling in hematopoiesis and lymphopoiesis. Cell Death Dis 2015;6:e1981. [Crossref] [PubMed]
- Bennett LB, Taylor KH, Arthur GL, et al. Epigenetic regulation of WNT signaling in chronic lymphocytic leukemia. Epigenomics 2010;2:53-70. [Crossref] [PubMed]
- Chim CS, Fung TK, Wong KF, et al. Infrequent Wnt inhibitory factor-1 (Wif-1) methylation in chronic lymphocytic leukemia. Leuk Res 2006;30:1135-9. [Crossref] [PubMed]
- Chim CS, Pang R, Fung TK, et al. Epigenetic dysregulation of Wnt signaling pathway in multiple myeloma. Leukemia 2007;21:2527-36. [Crossref] [PubMed]
- Román-Gómez J, Cordeu L, Agirre X, et al. Epigenetic regulation of Wnt-signaling pathway in acute lymphoblastic leukemia. Blood 2007;109:3462-9. [Crossref] [PubMed]
- Pongracz J, Hare K, Harman B, et al. Thymic epithelial cells provide WNT signals to developing thymocytes. Eur J Immunol 2003;33:1949-56. [Crossref] [PubMed]
- Weerkamp F, Baert MR, Naber BA, et al. Wnt signaling in the thymus is regulated by differential expression of intracellular signaling molecules. Proc Natl Acad Sci U S A 2006;103:3322-6. [Crossref] [PubMed]
- Perry JM, He XC, Sugimura R, et al. Cooperation between both Wnt/{beta}-catenin and PTEN/PI3K/Akt signaling promotes primitive hematopoietic stem cell self-renewal and expansion. Genes Dev 2011;25:1928-42. [Crossref] [PubMed]
- Reya T, Duncan AW, Ailles L, et al. A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature 2003;423:409-14. [Crossref] [PubMed]
- Huang J, Nguyen-McCarty M, Hexner EO, et al. Maintenance of hematopoietic stem cells through regulation of Wnt and mTOR pathways. Nat Med 2012;18:1778-85. [Crossref] [PubMed]
Cite this article as: Staal FJ. Wnt signalling meets epigenetics. Stem Cell Investig 2016;3:38.


