
Novel Human Umbilical Di-Chimeric (HUDC) cell therapy for transplantation without life-long immunosuppression
Introduction
Remarkable advances in surgical techniques have allowed further development of the vascularized composite allotransplantation (VCA) field. However, there are limitations, such as the need for life-long immunosuppression to prevent allograft rejection, that preclude routine application of VCA in clinical practice. Over the past decade, different approaches were tested to induce tolerance and reduce side effects of immunosuppressive therapies (1-3).
Cell-based therapies are regarded as the most promising approach for tolerance induction in the VCA field (4-7). Thus, different sources of hematopoietic stem cells (HSCs), including bone marrow (BM), peripheral blood (PB), and umbilical cord blood (UCB)-derived progenitor cells have been tested to assess the impact of different cell lineage origins, dosages, and routes of cell delivery on the efficacy of cells engraftment, homing, regenerative quality, and tolerogenicity (8-10). Cell-based therapies were found to be more applicable for tolerance induction through chimerism and regulation of the alloreactive responses when compared to other alternative treatments (11).
UCB became a feasible option for pediatric and adult patients requiring allogeneic HSC transplantation when no suitable donor is available. UCB gained a status of an attractive stem cell source due to the accessibility and a lower immunogenicity, which may be associated with a higher number of immature lymphocytes as well as the presence of suppressor cells, such as mesenchymal stem cells (12).
Some preclinical studies confirmed improvement in the organ recovery using UCB-derived cells in large animal models of myocardial infarction (13-15). Furthermore, encouraging results of clinical application of UCB in bone marrow transplantation (BMT) opened new indications for UCB-derived cell therapies in the field of regenerative medicine including VCA transplants (16-20). One of the advantages of the UCB transplants (UCBTs) in VCA is the lower number of required human leukocyte antigen (HLA) matches, such as 5/6 or 6/6, compared to 10/10 required for the BMT match. Infusions of 4/6 or 5/8 HLA-matched UCB-derived cells are regularly performed in clinical cases where there is no access to the matched unrelated donor (21). Moreover, UCB transplantation has been associated with decreased risk of graft-versus-host disease (GvHD) compared to BMT (12). Retrospective published data demonstrate viable results regarding the use of unrelated UCBTs for hematological malignancies (22), inherited metabolic storage diseases (23), and primary immunodeficiency diseases (24) in the pediatric population. A cohort study, based on the American and European data records, confirmed that survival rates after the UCBT in the adult population were equivalent across the registries (25). Therefore, considering the unique properties of UCB, the creation of a novel UCB-based therapy would introduce a promising approach for the field of BMT, solid organ transplants (SOTs), and VCA transplants, due to the immunomodulatory properties of the UCBT when compared with the BMT (26).
Based on our twenty years of experience in studies on chimerism and tolerance induction in transplantation (27-35), we have recently reported the new generation of BM-derived CD34+ human hematopoietic chimeric cell (HHCC) via ex vivo polyethylene glycol (PEG)-mediated fusion (36). Moreover, we confirmed HHCC safety by the confirmation of the viability by Trypan Blue staining and LIVE/DEAD® assay, a low apoptosis profile by Annexin V staining and TUNEL assay, and the donor-specific genotype by lymphocytotoxicity test and STR-PCR analyses, as well as the proliferative properties by colony forming unit (CFU) assay and the immunogenicity by mixed lymphocyte reaction (MLR) of this new hematopoietic cell line.
To further assess the role of cells of hematopoietic origin in tolerance induction in transplantation, in the current study, we have successfully adapted our well-established ex vivo PEG-mediated fusion protocol to create Human Umbilical Di-Chimeric (HUDC) cells from two unrelated human UCB donors and tested in vitro HUDC cells’ properties as a potential novel supportive cell-based therapy for BMT, SOT, and VCA transplants. First, the successful creation of the HUDC cell line by ex vivo PEG-mediated fusion was confirmed. Next, we characterized the HUDC cells’ genotype by HLA class I, HLA class II, and short tandem repeat (STR) typings, and confirmed the presence of the alleles and loci specific for both human UCB donors. We have also confirmed the safety, high viability, and low apoptosis level of the HUDC cell therapy. Finally, the phenotype analysis confirmed the hematopoietic origin of HUDC cells, while the clonogenic properties were confirmed by CFU assay and the tolerogenic properties by MLR assay.
The creation of HUDC cell-based therapy will introduce the unique concept of personalized immunomodulatory supportive therapy to the transplantation field, thus opening a new era of immunosuppression free transplantation for BMT, SOT, and VCA transplants. We present this article in accordance with the MDAR reporting checklist (available at https://sci.amegroups.com/article/view/10.21037/sci-2023-024/rc).
Methods
Creation of the new HUDC cell line from UCB cells
UCB cells isolation
For each experiment, the human UCB units were purchased from Cleveland Cord Blood Bank. The UIC Office for the Protection of Research Subjects has determined that this activity does not meet the definition of human subjects’ research as defined by the 45 Code of Federal Regulations (CFR) 46.102(f). No ethical approval or informed consent was required due to the nature of this study. The human UCB cells were isolated from UCB derived from unrelated male and female donors using density gradients (Lymphoprep™, StemCell Technologies, Vancouver, Canada). The UCB samples were centrifuged for 25 min at 300 g, and the cells in interphase were collected. Next, the UCB cells were purified using anti-human CD235a (glycophorin A) MicroBeads and magnetic-activated cell sorting (MACS) separation (Miltenyi Biotec, Bergisch Gladbach, Germany) according to manufacturer’s instructions. Then, the isolated UCB cells were washed in RPMI medium containing 10% fetal bovine serum (FBS) and 1× antibiotic/antimycotic solution and resuspended for counting.
PEG-mediated cell fusion procedure
The HUDC cell line was created from human UCB cells derived from two unrelated donors (donors 1 and 2) (Figure 1A). The unstained parent UCB cells were collected, isolated, and fluorescently stained separately for each donor with PKH26-red or PKH67-green traceable cell membrane dyes (MiliporeSigma, Burlington, MA, USA). First, the pellets with the UCB parent cells were suspended in diluent C (Sigma, St. Louis, MO, USA), and 6 µL of each PKH dye was added into 1 mL of total volume. The PKH staining was performed for 3 min. Next, the PKH26- and PKH67-stained UCB cells were washed and suspended in serum-free RPMI 1640 medium (Thermo Fisher Scientific, Waltham, MA, USA), mixed in a ratio of 1:1, and fused using 500 µL of PEG 4000 solution (EMD, Burlington, MA, USA). The cell fusion procedure was performed sixteen times as previously reported (37,38). Fused HUDC cells were centrifuged, and suspended in DPBS-based fluorescence-activated cell sorting (FACS) buffer containing 25 mM HEPES, 2 mM ethylenediaminetetraacetic acid (EDTA) and 1% FBS in preparation for sorting (BD FACSAriaTM II cell sorter, Becton Dickinson, Franklin Lakes, NJ, USA) based on PKH26-red and PKH67-green fluorescent cell labeling. Double-stained PKH26/PKH67 cells, representing the HUDC cells, were selected and subjected to further analysis. The purity of the created HUDC cells (1×105 cells, n=3) was assessed by BD LSRFortessaTM cell analyzer (BD Biosciences, San Jose, CA, USA). The samples were collected for flow cytometry (FC) and confocal microscopy (CM) analyses. Finally, the sorted HUDC cells were seeded in low-adhesion culture T25 or T75 flasks at a minimum density of 105 cells/mL in an optimized medium (H3000 + CD34 supplement + 20% FBS, StemCell Technologies, Vancouver, Canada).
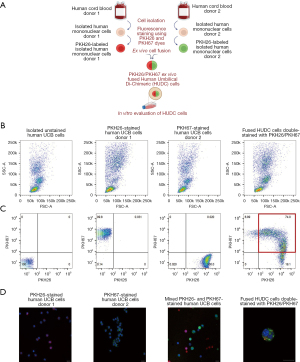
FC and CM analysis for confirmation of HUDC cell fusion
The creation of the HUDC cells by ex vivo PEG-mediated cell fusion of human UCB from two unrelated donors was confirmed by the presence of the double-stained cells (orange), characteristic for the overlap of PKH26-red and PKH67-green fluorescent dyes.
For CM assessment, all collected samples of the PKH26- and PKH67-stained human UCB donor cells (donors 1 and 2, respectively), the mixed unfused PKH-stained human UCB cells, and the fused HUDC cells were spun onto positively charged lysine coated microscope slides, fixed in 4% paraformaldehyde (EMS, Hatfield, PA, USA) for 15 min in room temperature, mounted with VECTASHIELD® Antifade Mounting Medium with DAPI (Vector Laboratories, Burlingame, CA, USA), and examined on a Leica TCS SP upright confocal microscope with Retiga 2000R camera (True Confocal Scanner Leica, Wetzlar, Germany) and ImagePro Plus (Media Cybernetics, Rockville, MD, USA).
Genotype analysis of HUDC cells by lymphocytotoxicity test for HLA class I and II typing and STR-polymerase chain reaction (STR-PCR)
The HLA class I and II typing was performed using the UCB fusion parent cells and HUDC cells immediately after the fusion procedure. The UCB and HUDC cell suspensions were incubated for 20 min with Lympho-Kwik T Lymphocyte reagent (One Lambda Inc., Canoga Park, CA, USA) to isolate T lymphocytes, while B lymphocytes were purified using Dynabead HLA Cell Prep II (Invitrogen, Waltham, MA, USA) according to the manufacturer instructions. Next, T and B lymphocytes were applied on commercially available class I and II HLA typing trays (Jena Bioscience GmbH, One Lambda Inc.). Trays were examined under an inverted fluorescence microscope (Leica, Germany).
The STR-PCR analysis was performed after completion of the fusion procedure to determine the presence of the loci for all STRs specific for both human UCB parents cell donors, evaluate genetic identity, and confirm chimerism in the created HUDC population. The DNA samples from the UCB parent cells and HUDC cells were extracted using the DNeasy Blood and Tissue Isolation kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. The STR-PCR analysis (n=4) was performed as previously described (36). The extracted DNA was amplified using Biosystems ABI 3730 DNA Analyzer (Thermo Fisher Scientific) and AmpFLSTRTM IdentifilerTM PCR Amplification kit (Thermo Fisher Scientific). Appropriate negative and positive controls were used. Data was obtained for the following STR loci (genetic markers): D8S1179, D21S11, D7S820, CFS1PO, D3S1358, TH01, D13S317, D16S539, D2S1338, D19S433, vWA, TPOX, D18S51, AMEL, D5S818, FGA. The raw data were uploaded to the GeneMapperTM 5.0 analysis software (Thermo Fisher Scientific) and the allelic profiles were created according to the analysis conditions supplied by Promega (Madison, WI, USA). The STR-based chimerism assessment was performed using Excel application to compute the ratio of donor specific alleles present in the DNA isolated from the HUDC cells.
FC analysis for assessment of HUDC cells’ phenotype
To assess the phenotype of the fused HUDC cells, immunostaining was performed at 7 days after fusion to characterize the cell surface marker expression. Assessments of the following hematopoietic markers were evaluated for the expression of T lymphocytes (CD3, CD4, CD8), B lymphocytes (CD5, CD19), and stem cells (CD34). The following anti-human monoclonal antibodies were used: CD3 (APC, 300311, RRID AB_314047, BioLegend, San Diego, CA, USA), CD4 (APC VioBlue® REAfinityTM, Miltenyi Biotec, 130-114-725, Gaithersburg, MD, USA), CD5 (APC/Cyanine7, 300629, RRID AB_2566472, BioLegend), CD8 (Brilliant Violet 421TM, 344747, RRID AB_2629583, BioLegend), CD19 (Pacific BlueTM, 115526, RRID AB_493341, BioLegend), and CD34 (BD PharmingenTM APC, 561209, RRID AB_10683161, BD Biosciences). The UCB controls and HUDC cells samples (1×106, n=3/group) were blocked with human BD Fc BlockTM Reagent (BD Biosciences) for 5 min and incubated with the previously mentioned antibodies, added at saturating concentrations into PBS solution containing 1% bovine serum albumin (BSA). Assessment was performed using BD® LSR II Flow cytometer (BD Biosciences) and FlowjoTM (Becton Dickinson).
LIVE/DEAD® assay for assessment of HUDC cells’ viability and Annexin V staining for apoptosis level
The viability of the UCB donor cells before fusion as well as the viability of the created HUDC cells at 3 hours after fusion (n=3) was assessed by LIVE/DEAD® Fixable Dead Cell staining (Thermo Fisher Scientific) according to the manufacturer’s instructions. The apoptosis assessment of the unstained human UCB cells, PKH-stained UCB cells, and HUDC cells (1×106, n=3/group) was performed using Annexin V-APC staining (BioLegend) according to the manufacturer’s instructions (BioLegend). The results were evaluated by LSRFortessaTM (BD Biosciences) and FlowjoTM software (Becton Dickinson). Gating strategy for Annexin V/Sytox Blue staining was completed as previously described (35).
Single cell gel electrophoresis (SCGE) COMET assay for assessment of HUDC cell fusion safety
The SCGE COMET assay (Abcam, Cambridge, United Kingdom) was performed to assess the DNA damage after cell fusion (n=3). The UCB donor cells and HUDC cells samples were processed according to the manufacturer’s instructions. The UCB donor cells and HUDC cells samples were visualized immediately using Vista Green fluorescent dye diluted in Tris-EDTA (TE) buffer (1:10,000), and examined under the MZ16FA stereomicroscope (Leica) equipped with a Retiga 2000R camera (True Confocal Scanner Leica). The DNA damage was confirmed by the visual presence of the ‘comet’-like tail which refers to the pattern of damaged DNA migrating through the electrophoresis gel. With the visual scoring system, a total of fifty cells were evaluated on each electrophoresis gel, and the DNA damage was classified into five categories from 0 (no tail) to 4 (all DNA in tail) with the average minimal score of 0 arbitrary unit and average maximal score of 200 arbitrary units (39).
CFU assay for assessment of HUDC cells’ clonogenic properties, proliferation, and differentiation
To confirm maintenance of the clonogenic properties, proliferation, and differentiation of the created HUDC cells, the samples of the UCB donor cells, the PKH26- and PKH67-stained mixed UCB cells, and the fused HUDC cells were analyzed after 14 days of cell culture. First, the UCB cells and the fused HUDC cells were cultured separately, and seeded in 35 mm dishes according to the MethoCult® manufacturer’s instructions (StemCell Technologies). Next, 1×103 cells were plated on a methylcellulose-based medium (MethoCult® H4034, StemCell Technologies). Following CFU assay, cell colony numbers were evaluated under the light microscope (Leica MZ16FA stereomicroscope, Leica) equipped with a Leica DFC290 HD Color Digital Camera, and presented in a chart for further analysis. Photographs of the cell colonies were taken with the high objective lens (×40).
MLR assay for assessment of HUDC cells’ tolerogenic properties, and immunogenicity
PB T-cells derived from one of the UCB donor cells (n=3/assay) were labeled with 3 µM CellTrace™ Violet (Thermo Fisher Scientific) and applied as responder cells (1×106/assay). The UCB cells and the created HUDC cells were irradiated (25 Gy) using a 137Cs source (Gammacell® 3000, Ottawa, ON, Canada) and used as stimulator cells. The HUDC cells were seeded at concentration of 0.5×106, 1×106 and 2×106 cells/assay. The mixed responder and stimulator cells were cultured in 200 µL of “complete” RPMI 1640 medium containing 10% FBA and 1X antibiotic/antimycotic solution (1XAAS, Thermo Fisher Scientific) for 5 days.
Statistical analysis
Statistical analysis was performed using Minitab software (OriginLab Corp. Northampton, MA, USA). Assays were performed in independent experiments with isolated unstained and/or PKH-stained human UCB donor cells as reference controls. Values are presented as mean ± standard deviation. Statistical differences between respective groups were assessed using one-way analysis of variance (ANOVA) followed by Tukey’s post-hoc test. Statistical significance was considered at P<0.05.
Results
Confirmation of a new HUDC cell line creation from two unrelated UCB donors
The study design of HUDC cells creation by ex vivo PEG-mediated cell fusion of the human UCB cells derived from two unrelated donors (donors 1 and 2) is presented on Figure 1A. The creation of the HUDC cell line was confirmed by FC (Figure 1B,1C) and CM (Figure 1D). The average fusion efficacy assessed as presented on Figure 1C (right image, red gate) was at 67.4%±3.4% (n=5). Additionally, the UCB cells of donor 1 stained with PKH26-red fluorescent dye and the UCB cells of donor 2 stained with PKH67-green fluorescent dye were analyzed by CM. Before the fusion procedure, the donor cells were mixed and CM assessments revealed the absence of PKH fluorescent dyes overlap. The double-stained PKH26/PKH67 HUDC cells of yellow color (overlapping of PKH26-red and PKH67-green traceable cell membrane dyes) confirmed the efficacy of the fusion procedure of the human UCB cells from two unrelated donors and the chimeric state of the created HUDC cells. We confirmed the successful creation of HUDC cells from two unrelated human UCB donor cells by FC and CM analyses.
Confirmation of the donor-specific genotype in the HUDC cell line
The genotype of HUDC cells was assessed by lymphocytotoxicity test, confirming the presence of HLA class I and II antigens (A, B, Cw, DR, DRB3, DRB4, DQA, DQB) specific for both unrelated human UCB donor cells (donors 1 and 2) after completion of cell fusion (Figure 2A). The STR-PCR assessment of HUDC cells confirmed the presence of the STR’s loci (genetic markers: D8S1179, D21S11, D7S820, CFS1PO, D3S1358, TH01, D13S317, D16S539, D2S1338, D19S433, vWA, TPOX, D18S51, AMEL, D5S818, FGA) specific for both unrelated human UCB donor cells (donors 1 and 2) after completion of cell fusion (Figure 2B). An overall donor chimerism was achieved at 49%±8.3% (n=4).
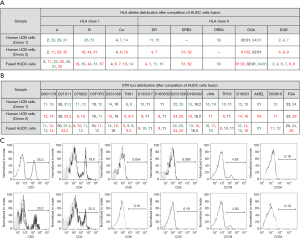
Confirmation of hematopoietic phenotype of HUDC cells by FC
The phenotype of the created HUDC cells was assessed by FC analysis at 7 days after cell fusion procedure (Figure 2C), and revealed a low number of CD8, CD5 and CD34 (<1%) positive cells within the untreated UCB control cells and HUDC cell population. The HUDC cells presented 20.2% of CD3 and 22.3% of CD4 positive cells which was comparable to the 25.3% of CD3 and 19.6% of CD4 expression on the surface of untreated UCB control cells. The expression of CD19, a marker specific for B lymphocytes lineage, was 4–5% on the surface of the isolated UCB control cells and HUDC cells. There was no significant change in expression of the assessed hematopoietic markers on the untreated UCB control cells before fusion procedure and on the created HUDC cells after the fusion procedure, further confirming that the ex vivo PEG-mediated fusion procedure has not introduced significant changes in the expression patterns of the assessed hematopoietic cell surface markers between the UCB control cells and HUDC cells.
Confirmation of high viability and low apoptosis in the created HUDC cell line
The viability of the HUDC cells was assessed by LIVE/DEAD® assay (Figure 3A). The results showed that, when compared to the negative control of unstained UCB cells presenting with 0% of dead cells (Figure 3A, left image), only 0.47% of the cells were dead in the HUDC cell population (Figure 3A, middle image) at 3 hours after cell fusion. The positive control of PKH-stained UCB cells treated with 500 µM of H2O2 (Figure 3A, right image) showed 97.4% of dead cells, indicating that the cell fusion procedure did not affect the viability of HUDC cells.
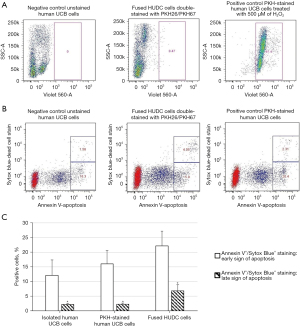
The assessment of apoptosis level in the HUDC cell population was performed by Annexin V/Sytox Blue assay (Figure 3B,3C). The results showed that, when compared to the negative control of unstained UCB cells presenting with 16.3% of apoptotic cells (Figure 3B, left image), only 15.9% of the cells were apoptotic in the HUDC cell population (Figure 3B, middle image) at 3 hours after cell fusion. The positive control of PKH-stained UCB cells before the fusion procedure (Figure 3B, right image) showed 20.4% of apoptotic cells, indicating that the staining procedure did not affect the apoptosis level of the fused HUDC cells. Annexin V+/Sytox Blue+ staining showed an increase in the average number of early apoptotic cells in the PKH26-stained UCB cells (16%±4.6%) and HUDC population (22.2%±5%) compared to isolated UCB controls (12.1%±5.2%); however, it was not statistically significant (P>0.05, Figure 3C). Annexin V+/Sytox Blue+ staining showed an increase in the number of late apoptotic HUDC cells after fusion (6.8%±2.1%), compared to isolated UCB cells and PKH-stained UCB cells (2.16%±0.4% and 2.3%±0.4%, respectively; P<0.05 vs. HUDC cells, Figure 3C).
Confirmation of fusion safety by lack of genotoxicity in the created HUDC cell line
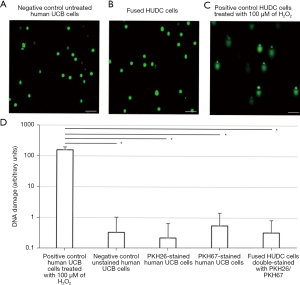
The safety of the cell fusion procedure was evaluated by the COMET assay (Figure 4) after the fusion procedure. The COMET assay analysis revealed the absence of DNA damage for the negative control of untreated UCB donor cells (Figure 4A) and fused HUDC cells (Figure 4B), as confirmed by the lack of a ‘comet’-like tail. Assessment of the positive control of HUDC cells treated with 100 µM of H2O2 (Figure 4C) revealed the presence of DNA damage, confirmed by the presence of a ‘comet’-like tail. These results confirm the absence of DNA damage in the created HUDC cells and the safety of the cell fusion procedure, as determined by the COMET assay. Visual scoring of COMET assay of the negative control of UCB cells, PKH26-stained UCB cells, PKH67-stained UCB cells, and HUDC cells showed negligible number of cells scored as 1 and 2 (0.3±0.7, 0.2±0.4, 0.6±0.9 and 0.3±0.5, respectively; P>0.05) compared to the positive control of UCB cells treated with 100 µM of H2O2 (158±37, P<0.05) (Figure 4D).
Confirmation of clonogenic properties of HUDC cell line by CFU assay
The clonogenic properties of the created HUDC cells were assessed by CFU assay at 3 hours after fusion procedure and 14 days of cell culture post-fusion. CFU assay confirmed that the fused HUDC cells produce the same types (Figure 5A) and comparable numbers of burst forming unit-erythroid (BFU-E)/colony forming unit-erythroid (CFU-E) and colony forming unit-granulocyte, macrophage (CFU-GM) colonies as the human UCB control cells (Figure 5B). The average total number of BFU-E/CFU-E colonies produced by: the isolated unstained UCB cells was 29.1±7.3, the mixed PKH-stained UCB cells was 33.9±14.3, and the fused HUDC cells was 21.4±8 (P>0.05). The average total number of CFU-GM colonies produced by the isolated unstained UCB cells was 25.4±4.7, the mixed PKH-stained UCB cells was 28.3±9.4, and the fused HUDC cells was 23.7±4.5 (P>0.05). Evaluation of culture of the isolated unstained UCB cells, the mixed PKH-stained UCB cells, and the created HUDC cells revealed a lack of statistically significant difference in the average number of BFU-E/CFU-E and CFU-GM among the analyzed groups. The comparison of the average number of colony forming unit-granulocyte, erythroid, macrophage, megakaryocyte (CFU-GEMM) colonies produced by the mixed PKH-stained UCB cells and the HUDC cells was not statistically significant (6.5±3.1 and 5.4±2.4, respectively; P>0.05); however a decrease in the number of CFU-GEMM colonies was observed in the PKH-stained UCB cells and the fused HUDC cells when compared to the isolated unstained UCB cells (P<0.01, Figure 5B).
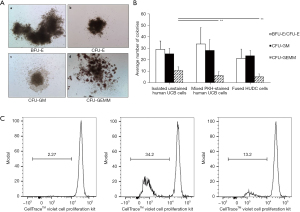
Confirmation of tolerogenic properties of HUDC cell line by MRL assay
The results showed that when compared to the negative control of unstimulated CellTrace™ Violet-labeled T-cell responders presenting with a proliferation rate of 2.27%±0.5% (Figure 5C, left image), the proliferative response of CellTrace™ Violet-labeled T-cell responder cells stimulated with irradiated allogeneic UCB cells was at 34.2%±6.9%, P<0.05 (Figure 5C, middle image). MLR assay showed significantly decreased response of CellTrace™ Violet-labeled T-cell to irradiated HUDC cells (13.2%±2.4%, P<0.05) (Figure 5C, right image) when compared to the allogeneic control of UCB stimulated responder T-cells.
Discussion
VCA is a pioneering and emerging field of reconstructive surgery, providing promising solutions for traumatic injuries in civilian and military patients. As we approach the fifteen-year anniversary marking the first near-total face transplant in the United States performed by our group (40), remarkable advances in surgical techniques have outpaced consistent and reproducible solutions to the challenge of inducing long-term recipient immune tolerance to the allogeneic transplants. Thus, VCA are considered as the life-enhancing rather than life-saving procedures due to the potential risk of life-long immunosuppression and related side effects. Potential candidates that could greatly benefit from VCA are currently disfavored by the prevailing necessity and associated morbidity of a life-long immunosuppression, known for severe side effects including: malignancy, metabolic disorders, opportunistic infections, as well as chronic rejection (1-3). The introduction of new, easily available cell-based therapies presenting a low immunogenic profile is critical to advance stem cell transplantation and successfully treat hematopoietic disorders and malignancies as well as facilitate tolerance induction in the setting of SOT, and VCA transplantation.
The most common approach to improve the efficacy of UCB transplantation is to increase the number of HSC via in vitro cell cultures (41); however, long-term culturing may decrease the expression of homing markers at the cells’ surface as well as capability of HSC to differentiate after transplant.
Decades of investigational work into the intentional immune system reprogramming to recognize allogeneic tissues as “self”, well known as the paradigmatic “Holy Grail” in immunology and transplantology, have elucidated the importance of introducing donor-specific hematopoietic chimerism as a foundational mechanism and a means to perform VCA without the need for life-long immunosuppression (42). As clinically observed, VCA graft rejection occurs by cytotoxic immune-cell mediated processes against HLA class I cell markers (43). Introduction of cell-based therapies, such as donor-recipient chimeric cells (DRCCs) of hematopoietic lineage origin, is regarded as the most promising approach towards induction of donor-specific immune tolerance.
In different animal models, immune tolerance induction was already successful via hematopoietic chimerism and was confirmed across the HLA barrier in VCA (5,44) and SOTs (45). Although tolerance induction in human allogeneic transplantation was not yet achieved, the examples of donor BM transplant supporting graft acceptance in human VCA (4,45) and SOT (46-49) have been encouraging and should be considered in the future trials.
The toxicity of the recipient conditioning required for marrow transplant limits the routine use of BMT in the clinical setting. Therefore, the myelosuppressive conditioning regimens should balance the potency required to sufficiently overcome the mismatched-HLA barrier, and at the same time, prevent severe impairment of the hematopoiesis. It is estimated that half of patients on the transplant list do not have the HLA-matched donor available despite the large number of over 13 million registered BM donors worldwide (50). This is exemplified by the fact that there are approximately 10 to 15 thousand patients per year that have trouble finding an unrelated adult donor due to the rapid disease progression and subsequent death (51).
Moreover, match disparities most significantly and disproportionately affect racial and ethnic minorities (52). To overcome the lack of matched BM donors, the use of different HSCs sources could greatly benefit patients seeking for different alternatives. Previous research into HSC sources, which include BM, PB and UCB-derived progenitors, illustrates the diversity of the hematopoietic lineage profiles including different sources of the cell origin (8-10).
The UCB-derived hematopoietic progenitors are very appealing to expand the donor pool. Since the advent of UCB banking in the late 1990s, it has been recorded that over 55 thousands cord blood units have been provided for transplantation purposes (53). The UCB is an attractive potential option over the sources of the HSCs derived from the unrelated donors of BM or PB for a variety of reasons including: a greater proportion of highly proliferative progenitor cells (54); a lower severity and incidence of acute GvHD compared to the other graft sources (50,55); a faster bank procurement with a 3-week waiting period for UCB units compared to 3-month for matched unrelated BM donor (56); a lower immune reactivity with immunomodulatory properties (57,58); and a lower stringency for HLA matching compared to BMT, which is currently clinically accepted at the threshold of 4 of 8 HLA antigens (HLA-A, -B, -C, and -DRB1) (59).
Over the past 20 years, our Microsurgery Laboratory developed different chimeric cell lines to overcome these limitations. First, the DRCCs were created by ex vivo PEG-mediated fusion of BM cells derived from two unrelated August Copenhagen Irish (ACI) and Lewis rats (34,35). The pro-tolerogenic properties of the created DRCCs were confirmed, facilitating DRCCs engraftment, prolonging VCA survival, and inducing donor-specific immune tolerance. After successful testing of DRCCs in vivo in the rat VCA model, the next step was to create the CD34+ HHCC for potential clinical applications in transplantation.
Inspired by the potential of the encouraging outcomes of the rat DRCCs, our Microsurgery Laboratory successfully created HHCC from the BM-derived CD34+ cells originating from two unrelated human donors, according to the previously described protocol (36). The properties of the HHCC originating from human CD34+ cells were characterized in vitro, confirming the tolerogenic properties of HHCC and the potential application of HHCC therapy for tolerance induction in BM, solid organs, and VCA transplantation (36).
Therefore, the fusion of HSCs across HLA barriers represents a novel approach for transplant tolerance induction with a great clinical potential. Considering the need to expand the application of HSCs in the search of the “universal donor” and based on the attractive qualities of low immunogenicity of UCB cells, we propose a new cellular therapy based on ex vivo created chimeric cells derived from UCB cells from two unrelated donors as an alternative approach to the BM-based therapies for the BM, solid organs, and VCA support.
The current study was designed to create human HUDC cells via fusion of UCB cells derived from two unrelated human donors, as a novel alternative therapy for tolerance induction in BMT, SOT, and VCA transplantation. We confirmed the feasibility, reproducibility, and safety of the ex vivo PEG-mediated fusion protocol applied for creation of HUDC cells. Next, we confirmed in vitro the hematopoietic phenotype as well as high viability, proliferative and clonogenic properties of HUDC cells.
The creation of HUDC cells relies on the fusion of the genetic material from the two unrelated human UCB donor cells through ex vivo PEG-mediated fusion procedure. Therefore, the created donor-recipient HUDC cells are carrying the HLA class I and II antigens and the STR loci specific for each of the human parent UCB donor cells, as confirmed by the lymphocytotoxicity test and STR-PCR analysis, respectively. Phenotype analysis at 7 days after cell fusion confirmed hematopoietic origin and stability of the HUDC cells’ phenotype. Since HUDC cells represent an example of the Di-Chimera created from the two unrelated donors, HUDC cells may reduce immune response towards the recipient and the need for immunosuppression.
The high viability of HUDC cells after cell fusion was comparable to the viability of the unstained human UCB cells before the fusion procedure. Additionally, the level of late signs of apoptosis of the fused HUDC cells was low when compared to the positive control of PKH-stained UCB cells before the fusion procedure performed by the Annexin V and Sytox Blue assays, further supported the stability of the new HUDC cell line.
To ensure the long-term safety of the HUDC cells therapy and the lack of genotoxicity of the ex vivo cell fusion procedure, the COMET assay was performed on the UCB donor cells before fusion and on the created HUDC cells after fusion, and revealed the absence of the ‘comet’-like tail, characteristic of the DNA damage, in the created HUDC cells, thus confirming further the safety of the ex vivo PEG-mediated fusion procedure.
The clonogenic properties were confirmed by the differentiation of HUDC cells into the granulocyte, erythroid, macrophage, and megakaryocyte progenitor cells after 14 days of cell culture. The formation of colonies in the cell culture further confirmed the hematopoietic origin of the created HUDC cell line. Moreover, the proliferative rate of the HUDC cells was comparable to the rate of the unfused human UCB cells after 14 days of incubation on the methylcellulose-based medium, confirming the proliferative properties of the fused HUDC cells. Finally, the MLR assay revealed a decreased immune response of the HUDC cells compared to allogeneic control, further verifying the tolerogenic properties of HUDC cells.
There are several similarities between our two hematopoietic lines—the HHCC and HUDC cell line of created human chimeric cells. Both lines represent cells of the human hematopoietic lineage origin and both contain rich sources of the HSCs applicable for transplantation (36). When comparing the hematopoietic markers, the UCB cells have been shown to contain a high percentage of primitive stem cells, including very small embryonic-like stem cells, and a lower immunogenicity compared to other sources of stem cells, such as BM and PB (60,61). Moreover, HSCs derived from BM or PB have been extensively studied and characterized for the presence of hematopoietic markers, including expression of CD34, CD38, and CD133. It is also well established that HSC are capable of self-renewal and differentiation into various blood cell lineages (8-10). Regarding comparison of safety, it is well-known that the transplantation of UCB cells has been associated with a decreased risk of GvHD compared to BMT in addition to the significantly better immune profile when considering the donor match which is acceptable at the 4–6/10 for UCB and 10/10 for BM transplant (21). The safety of HHCC, which represent a new generation of BM-derived CD34+ HSC created via ex vivo PEG-mediated fusion, has been confirmed in our recently published study reporting the lack of DNA damage confirmed by COMET assay as well as a low apoptosis profile and a lack of tumor formation assessed by magnetic resonance imaging (MRI) (36).
In summary, the current study confirmed the feasibility and reproducibility of creation of a new hematopoietic HUDC cell line from human UCB cells derived from two unrelated donors via ex vivo PEG-mediated cell fusion procedure. Moreover, the chimeric state of HUDC cells was confirmed by the presence of HLA class I alleles, class II alleles, and the STR loci from both UCB donors whereas absence of genotoxicity was confirmed by the COMET assay. Furthermore, we demonstrated preservation of the hematopoietic phenotype and clonogenic properties of the HUDC cells under standard ex vivo cell culture conditions after cell fusion.
To the best of our knowledge, this is the first report on the successful creation of the new HUDC cell line via ex vivo PEG-mediated fusion of UCB cells derived from two unrelated human donors. This study characterized and confirmed in vitro the safety of ex vivo fused HUDC cells. This study suggests that HUDC cell line-based therapy may be a promising alternative approach to the BM-based therapies applied for tolerance induction in transplantation. The potential of donor-specific tolerance induction has promising implications for reducing the side effects of immunosuppressive protocols and addressing the problem of donor shortage in BMT. Furthermore, the possibility of creating personalized HUDC cell therapy that closely matches the HLA of the donor and the recipient offers a potential solution to the issue of the donor HLA incompatibility and the donor shortage.
In order to advance this therapy to clinical trials and facilitate potential therapeutic applications, our Microsurgery Laboratory aimed to characterize and confirm in vivo the safety and efficacy of the created HUDC cell therapy.
Conclusions
In this study, we successfully established the ex vivo fusion protocol to create a novel HUDC hematopoietic cell line from the UCB cells derived from two unrelated human donors. Creation of HUDC cells was confirmed by FC and CM. COMET assay confirmed lack of DNA damage and safety of ex vivo PEG-mediated cell fusion procedure. The unique concept of HUDC cell therapy represents a new promising approach for tolerance induction and immunosuppression for free transplants of solid organs, BM, and VCA.
Acknowledgments
The authors thank Natalia Filipek, MD (Department of Orthopaedics, University of Illinois at Chicago, Chicago, IL, USA), Medhat Askar, MD, PhD (Allogen Laboratories, Cleveland Clinic, Cleveland, OH, USA), for the technical support as well as staff members of Core Imaging Facilities at University of Illinois at Chicago for help with acquiring confocal microscopy images, and Flow Cytometry Core for technical assistance with HUDC cells sorting and acquisition of flow cytometry data. The authors would like to thank the BioRender service which was utilized to create Figure 1A.
Funding: This work was supported by the Army, Navy, NIH, Air Force, VA and Health Affairs to support the AFIRM II effort under Award No. W81XWH-13-2-0053, and the U.S. Army Medical Research Acquisition Activity, 820 Chandler Street, Fort Detrick MD 21702-5014.
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at https://sci.amegroups.com/article/view/10.21037/sci-2023-024/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://sci.amegroups.com/article/view/10.21037/sci-2023-024/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The UIC Office for the Protection of Research Subjects has determined that this activity does not meet the definition of human subjects’ research as defined by 45 CFR 46.102(f). No ethical approval or informed consent is required because of the nature of this study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Gordon CR, Avery RK, Abouhassan W, et al. Cytomegalovirus and other infectious issues related to face transplantation: specific considerations, lessons learned, and future recommendations. Plast Reconstr Surg 2011;127:1515-23. [Crossref] [PubMed]
- Petruzzo P, Dubernard JM. The International Registry on Hand and Composite Tissue allotransplantation. Clin Transpl 2011;247-53. [PubMed]
- Messner F, Etra JW, Dodd-O JM, et al. Chimerism, Transplant Tolerance, and Beyond. Transplantation 2019;103:1556-67. [Crossref] [PubMed]
- Schneeberger S, Gorantla VS, Brandacher G, et al. Upper-extremity transplantation using a cell-based protocol to minimize immunosuppression. Ann Surg 2013;257:345-51. [Crossref] [PubMed]
- Mathes DW, Chang J, Hwang B, et al. Simultaneous transplantation of hematopoietic stem cells and a vascularized composite allograft leads to tolerance. Transplantation 2014;98:131-8. [Crossref] [PubMed]
- Moris D, Cendales LC. Sensitization and Desensitization in Vascularized Composite Allotransplantation. Front Immunol 2021;12:682180. [Crossref] [PubMed]
- Yang JH, Johnson AC, Colakoglu S, et al. Clinical and preclinical tolerance protocols for vascularized composite allograft transplantation. Arch Plast Surg 2021;48:703-13. [Crossref] [PubMed]
- Moretta F, Petronelli F, Lucarelli B, et al. The generation of human innate lymphoid cells is influenced by the source of hematopoietic stem cells and by the use of G-CSF. Eur J Immunol 2016;46:1271-8. [Crossref] [PubMed]
- Berglund S, Magalhaes I, Gaballa A, et al. Advances in umbilical cord blood cell therapy: the present and the future. Expert Opin Biol Ther 2017;17:691-9. [Crossref] [PubMed]
- Zhu X, Tang B, Sun Z. Umbilical cord blood transplantation: Still growing and improving. Stem Cells Transl Med 2021;10:S62-74. [Crossref] [PubMed]
- Anggelia MR, Cheng HY, Lai PC, et al. Cell therapy in vascularized composite allotransplantation. Biomed J 2022;45:454-64. [Crossref] [PubMed]
- Han P, Hodge G, Story C, et al. Phenotypic analysis of functional T-lymphocyte subtypes and natural killer cells in human cord blood: relevance to umbilical cord blood transplantation. Br J Haematol 1995;89:733-40. [Crossref] [PubMed]
- Lim M, Wang W, Liang L, et al. Intravenous injection of allogeneic umbilical cord-derived multipotent mesenchymal stromal cells reduces the infarct area and ameliorates cardiac function in a porcine model of acute myocardial infarction. Stem Cell Res Ther 2018;9:129. [Crossref] [PubMed]
- Matar AJ, Crepeau RL, Mundinger GS, et al. Large Animal Models of Vascularized Composite Allotransplantation: A Review of Immune Strategies to Improve Allograft Outcomes. Front Immunol 2021;12:664577. [Crossref] [PubMed]
- Raposo L, Lourenço AP, Nascimento DS, et al. Human umbilical cord tissue-derived mesenchymal stromal cells as adjuvant therapy for myocardial infarction: a review of current evidence focusing on pre-clinical large animal models and early human trials. Cytotherapy 2021;23:974-9. [Crossref] [PubMed]
- Damien P, Allan DS. Regenerative Therapy and Immune Modulation Using Umbilical Cord Blood-Derived Cells. Biol Blood Marrow Transplant 2015;21:1545-54. [Crossref] [PubMed]
- Cwykiel J, Rafidi G, Siemionow M. Characterization of Ex Vivo Created Human Multi-Chimeric Cells - A Novel Approach for Tolerance Induction in Vascularized Composite Allotransplantation. Transplantation 2018;102:S720. [Crossref]
- Van SY, Noh YK, Kim SW, et al. Human umbilical cord blood mesenchymal stem cells expansion via human fibroblast-derived matrix and their potentials toward regenerative application. Cell Tissue Res 2019;376:233-45. [Crossref] [PubMed]
- Allan DS. Using umbilical cord blood for regenerative therapy: Proof or promise? Stem Cells 2020;38:590-5. [Crossref] [PubMed]
- Um S, Ha J, Choi SJ, et al. Prospects for the therapeutic development of umbilical cord blood-derived mesenchymal stem cells. World J Stem Cells 2020;12:1511-28. [Crossref] [PubMed]
- Dumont-Lagacé M, Feghaly A, Meunier MC, et al. UM171 Expansion of Cord Blood Improves Donor Availability and HLA Matching For All Patients, Including Minorities. Transplant Cell Ther 2022;28:410.e1-410.e5. [Crossref] [PubMed]
- Vairy S, Louis I, Vachon MF, et al. Intrabone infusion for allogeneic umbilical cord blood transplantation in children. Bone Marrow Transplant 2021;56:1937-43. [Crossref] [PubMed]
- Gabelli M, Veys P, Chiesa R. Current status of umbilical cord blood transplantation in children. Br J Haematol 2020;190:650-83. [Crossref] [PubMed]
- Smith AR, Gross TG, Baker KS. Transplant outcomes for primary immunodeficiency disease. Semin Hematol 2010;47:79-85. [Crossref] [PubMed]
- Cohen YC, Scaradavou A, Stevens CE, et al. Factors affecting mortality following myeloablative cord blood transplantation in adults: a pooled analysis of three international registries. Bone Marrow Transplant 2011;46:70-6. [Crossref] [PubMed]
- Wang M, Yang Y, Yang D, et al. The immunomodulatory activity of human umbilical cord blood-derived mesenchymal stem cells in vitro. Immunology 2009;126:220-32. [Crossref] [PubMed]
- Siemionow M, Ortak T, Izycki D, et al. Induction of tolerance in composite-tissue allografts. Transplantation 2002;74:1211-7. [Crossref] [PubMed]
- Siemionow M, Zielinski M, Ozmen S, et al. Intraosseus transplantation of donor-derived hematopoietic stem and progenitor cells induces donor-specific chimerism and extends composite tissue allograft survival. Transplant Proc 2005;37:2303-8. [Crossref] [PubMed]
- Siemionow MZ, Klimczak A, Unal S. Different routes of donor-derived hematopoietic stem cell transplantation for donor-specific chimerism induction across MHC barrier. Transplant Proc 2005;37:62-4. [Crossref] [PubMed]
- Siemionow M, Klimczak A. Chimerism-based experimental models for tolerance induction in vascularized composite allografts: Cleveland clinic research experience. Clin Dev Immunol 2013;2013:831410. [Crossref] [PubMed]
- Hivelin M, Klimczak A, Cwykiel J, et al. Immunomodulatory Effects of Different Cellular Therapies of Bone Marrow Origin on Chimerism Induction and Maintenance Across MHC Barriers in a Face Allotransplantation Model. Arch Immunol Ther Exp (Warsz) 2016;64:299-310. [Crossref] [PubMed]
- Siemionow M, Rampazzo A, Gharb BB, et al. The reversed paradigm of chimerism induction: Donor conditioning with recipient-derived bone marrow cells as a novel approach for tolerance induction in vascularized composite allotransplantation. Microsurgery 2016;36:676-83. [Crossref] [PubMed]
- Zor F, Bozkurt M, Cwykiel J, et al. The effect of thymus transplantation on donor-specific chimerism in the rat model of composite osseomusculocutaneous sternum, ribs, thymus, pectoralis muscles, and skin allotransplantation. Microsurgery 2020;40:576-84. [Crossref] [PubMed]
- Cwykiel J, Jundzill A, Klimczak A, et al. Donor Recipient Chimeric Cells Induce Chimerism and Extend Survival of Vascularized Composite Allografts. Arch Immunol Ther Exp (Warsz) 2021;69:13. [Crossref] [PubMed]
- Cwykiel J, Madajka-Niemeyer M, Siemionow M. Development of Donor Recipient Chimeric Cells of bone marrow origin as a novel approach for tolerance induction in transplantation. Stem Cell Investig 2021;8:8. [Crossref] [PubMed]
- Siemionow M, Brodowska S, Różczka K, et al. Creation of human hematopoietic chimeric cell (HHCC) line as a novel strategy for tolerance induction in transplantation. Stem Cell Investig 2022;9:11. [Crossref] [PubMed]
- Siemionow M, Cwykiel J, Heydemann A, et al. Creation of Dystrophin Expressing Chimeric Cells of Myoblast Origin as a Novel Stem Cell Based Therapy for Duchenne Muscular Dystrophy. Stem Cell Rev Rep 2018;14:189-99. [Crossref] [PubMed]
- Siemionow M, Cwykiel J, Marchese E, et al. A Novel Human Myoblast Chimeric Cells Therapy for Restoration of Muscle Function. Transplantation 2018;102:S355. [Crossref]
- Collins AR. The comet assay for DNA damage and repair: principles, applications, and limitations. Mol Biotechnol 2004;26:249-61. [Crossref] [PubMed]
- Siemionow M, Papay F, Alam D, et al. Near-total human face transplantation for a severely disfigured patient in the USA. Lancet 2009;374:203-9. [Crossref] [PubMed]
- Park YS, Lee Y, Choi NY, et al. Enhancement of proliferation of human umbilical cord blood-derived CD34(+) hematopoietic stem cells by a combination of hyper-interleukin-6 and small molecules. Biochem Biophys Rep 2022;29:101214. [Crossref] [PubMed]
- Zuber J, Sykes M. Mechanisms of Mixed Chimerism-Based Transplant Tolerance. Trends Immunol 2017;38:829-43. [Crossref] [PubMed]
- Etra JW, Raimondi G, Brandacher G. Mechanisms of rejection in vascular composite allotransplantation. Curr Opin Organ Transplant 2018;23:28-33. [Crossref] [PubMed]
- Leonard DA, Kurtz JM, Mallard C, et al. Vascularized composite allograft tolerance across MHC barriers in a large animal model. Am J Transplant 2014;14:343-55. [Crossref] [PubMed]
- Mathes DW, Randolph MA, Solari MG, et al. Split tolerance to a composite tissue allograft in a swine model. Transplantation 2003;75:25-31. [Crossref] [PubMed]
- Hequet O, Morelon E, Bourgeot JP, et al. Allogeneic donor bone marrow cells recovery and infusion after allogeneic face transplantation from the same donor. Bone Marrow Transplant 2008;41:1059-61. [Crossref] [PubMed]
- Ciancio G, Miller J, Garcia-Morales RO, et al. Six-year clinical effect of donor bone marrow infusions in renal transplant patients. Transplantation 2001;71:827-35. [Crossref] [PubMed]
- Leventhal J, Abecassis M, Miller J, et al. Chimerism and tolerance without GVHD or engraftment syndrome in HLA-mismatched combined kidney and hematopoietic stem cell transplantation. Sci Transl Med 2012;4:124ra28. [Crossref] [PubMed]
- Leventhal JR, Ildstad ST. Tolerance induction in HLA disparate living donor kidney transplantation by facilitating cell-enriched donor stem cell Infusion: The importance of durable chimerism. Hum Immunol 2018;79:272-6. [Crossref] [PubMed]
- Smith AR, Wagner JE. Alternative haematopoietic stem cell sources for transplantation: place of umbilical cord blood. Br J Haematol 2009;147:246-61. [Crossref] [PubMed]
-
World Marrow Donor Association - van Walraven SM, Brand A, Bakker JN, et al. The increase of the global donor inventory is of limited benefit to patients of non-Northwestern European descent. Haematologica 2017;102:176-83. [Crossref] [PubMed]
- World Marrow Donor Association, cord blood transplantation. Available online: https://wmda.info/cord-blood/basics-cord-blood/
- Broxmeyer HE, Hangoc G, Cooper S, et al. Growth characteristics and expansion of human umbilical cord blood and estimation of its potential for transplantation in adults. Proc Natl Acad Sci U S A 1992;89:4109-13. [Crossref] [PubMed]
- Rocha V, Labopin M, Sanz G, et al. Transplants of umbilical-cord blood or bone marrow from unrelated donors in adults with acute leukemia. N Engl J Med 2004;351:2276-85. [Crossref] [PubMed]
- Mogul MJ. Unrelated cord blood transplantation vs matched unrelated donor bone marrow transplantation: the risks and benefits of each choice. Bone Marrow Transplant 2000;25:S58-60. [Crossref] [PubMed]
- Weiss ML, Anderson C, Medicetty S, et al. Immune properties of human umbilical cord Wharton's jelly-derived cells. Stem Cells 2008;26:2865-74. [Crossref] [PubMed]
- Yoo KH, Jang IK, Lee MW, et al. Comparison of immunomodulatory properties of mesenchymal stem cells derived from adult human tissues. Cell Immunol 2009;259:150-6. [Crossref] [PubMed]
- Gupta AO, Wagner JE. Umbilical Cord Blood Transplants: Current Status and Evolving Therapies. Front Pediatr 2020;8:570282. [Crossref] [PubMed]
- Pappa KI, Anagnou NP. Novel sources of fetal stem cells: where do they fit on the developmental continuum? Regen Med 2009;4:423-33. [Crossref] [PubMed]
- Broxmeyer HE, Douglas GW, Hangoc G, et al. Human umbilical cord blood as a potential source of transplantable hematopoietic stem/progenitor cells. Proc Natl Acad Sci U S A 1989;86:3828-32. [Crossref] [PubMed]
Cite this article as: Siemionow M, Cwykiel J, Chambily L, Gacek S, Brodowska S. Novel Human Umbilical Di-Chimeric (HUDC) cell therapy for transplantation without life-long immunosuppression. Stem Cell Investig 2023;10:16.


