Making gametes from pluripotent stem cells: embryonic stem cells or very small embryonic-like stem cells?
The successful differentiation of mouse embryonic stem (mES) cells into haploid spermatids was recently achieved in vitro and published by a group led by 3 first authors including Quan Zhou, Mei Wang and Yan Yuan from China (1). It took almost 45 years to finally reach this stage since mES cells were first reported by Evans and Kaufman (2). The haploid spermatids generated were used for intra-cytoplasmic sperm injection in mouse oocytes and resulted in viable and fertile pups. This success raises hope to treat infertile patients in future. However, it will take much more time since the scientists at present are stuck on the first step itself that involves converting human ES cells into primordial germ cells-like cells (PGCLCs). Just recently Surani’s group reported that SOX17 plays a crucial role in specifying human ES cells into PGCLCs (3). It is basically difficult to convert ES cells into PGCLCs in vitro since it involves epigenetic reprogramming in addition to altered gene expression. Once a robust protocol is established to obtain PGCLCs from ES cells, they can easily be differentiated further into gametes. Thus it will be a while before gametes are obtained starting with human ES cells as concluded recently (4,5).
However, Zhou et al. (1) have achieved a major advance starting with mES cells, in obtaining spermatids which undergo meiosis in vitro and later produce viable and fertile pups. Their approach to achieve this success was very systematic and involved 3 major steps. First step was to differentiate transgenic mES cell lines into PGCLCs. The cells were transgenic for fluorescence reporter proteins under the control of regulatory elements of germ cell markers. This allowed them to easily track differentiation of mES cells into PGCLCs expressing STELLA in vitro associated with possible erasure of imprinting of parentally imprinted genes Snrpn and H19. In the second step, they cultured the PGCLCs along with neonatal testicular somatic cells in the presence of FSH/bovine pituitary extract/testosterone. Meiosis was tracked elegantly in vitro by studying sequential expression of specific markers. Initially chromosomal synapsis and DNA double stranded breaks and their resolution by homologous recombination repair was tracked by studying expression of SPO11 and RAD51. Expression pattern of phosphorylated H2A histone family member X recapitulated meiosis progression as it was broadly distributed throughout the nucleus on D8 reflecting an association with double stranded breaks in DNA and later focal appearance on sex chromosomes suggested completion of synapsis. The nucleus showed expression of SYCP1 and SYCP3. Later an up-regulation of meiotic markers Dmc1, Stra8 and Sycp3 was observed by D10 followed by up-regulation of transcripts specific for haploid cells including Prm1, haprin and acrosin. The in vitro generated spermatids were used for ICSI and basically success to obtain live pups in their IVF program was 9% in normal mice compared to 2.8% from the spermatids obtained from mES cells. These results need to be replicated by independent groups.
This low rate of pregnancy outcome using mES cells derived gametes reflects a serious scientific hurdle before this work could be translated into a clinical setting and possibly reflects inappropriate epigenetic status of the spermatids obtained in culture. A similar success to obtain spermatids from human ES cells has been reported in the past by Moore’s group (6) but they could not test the derived spermatid further for ethical reasons. As mentioned above, manipulating epigenetic status of a cell in a controlled manner is difficult compared to modifying gene expression on exposure of cells to a cocktail of growth factors and cytokines. It is intriguing that similarly the pancreatic islet progenitors derived from human ES cells have a very different epigenetic status compared to adult pancreas (7). ES/iPS cells are falling short (possibly because of their epigenetic profile) in that they tend to give rise to their fetal counterparts (8-10) and may not be really useful to regenerate adult organs. This is the underlying reason why the field of ES/iPS cells for regenerating age-related diseases has not moved as was expected.
We had earlier discussed that rather than ES cells [obtained from the inner cell mass (ICM) of blastocyst-stage embryo], very small embryonic-like stem cells (VSELs) in adult gonads, which are equivalent to PGCs (primordial germ cells obtained from epiblast-stage embryo), may be better stem cell candidates to make gametes (11). Based on their ontogeny, VSELs are relatively more mature developmentally as well as epigenetically compared to ES cells and thus spontaneously differentiate into sperm (12) and eggs (13-15) in vitro. Similarly we have reported that VSELs spontaneously regenerate adult mouse pancreas after partial pancreatectomy (16). Compared to ES/iPS cells which are being exploited to yield islet progenitors in culture, VSELs have the ability to regenerate both acinar cells and islets in the pancreas. More importantly, VSELs will bring about endogenous regeneration which is much more physiological compared to ES/iPS cells which are being used to generate progenitors for cell therapy.
The VSELs exist in adult ovary (13-15) and testis (17-20) in addition to the tissue specific progenitors including ovary stem cells (OSCs) in ovary and spermatogonial stem cells (SSCs) in testis. VSELs survive oncotherapy in both mice (15,18) and human (21) gonads because of their quiescent nature whereas OSCs/SSCs and germ cells get destroyed since oncotherapy basically targets actively dividing cells. They are also reported in non-functional human gonads (13,20). These surviving, endogenous VSELs in azoospermic testis and POF ovary can be exploited to regenerate the non-functional gonad. We had envisaged that it is the compromised niche that does not allow the surviving VSELs to restore gonadal function after oncotherapy. Based on this, Anand et al. (18) restored spermatogenesis and sperm production in busulphan treated testis by transplanting niche (Sertoli or bone marrow derived mesenchymal) cells via inter-tubular route into the testicular interstitium.
A critique of the study (1) is that they did not observe mES cells giving rise to SSCs in culture. Rather the mES cells differentiated into PGCLCs which directly underwent meiosis to produce haploid spermatids. In contrast, we have recently reported for the first time that small-sized VSELs, that survive busulphan treatment in mouse testis, undergo asymmetric cell divisions to give rise to slightly bigger SSCs (22). A chemoablated testis serves as an excellent model to study stem cells biology. The SSCs in turn divide rapidly by symmetric cell divisions followed by clonal expansion to form cell aggregates (Figure 1). We had earlier shown that the testicular cells isolated from chemoablated testis on culture spontaneously differentiate into sperm (12). The Sertoli cells attach to the culture surface and provide a somatic support and act as a source of growth factors and cytokines required for stem cells differentiation. Conditioned medium collected from healthy Sertoli cells culture was used for the cultures supplemented with FSH (5 IU). Surprisingly no additional factors like activin or retinoic acid were added to induce the cells to undergo meiosis. At the end of 3 weeks culture we observed various stages of spermiogenesis, cytoplasmic drops being shed and appearance of mouse sperm with characteristic hook shaped head (Figure 1). On D7 we could detect Gfra, Dazl, Prohibitin and Protamine suggestive of presence of spermatogonial stem cells, germ cells, spermatocytes and sperm in culture and that the cells underwent meiosis resulting in expression of post-meiotic markers.
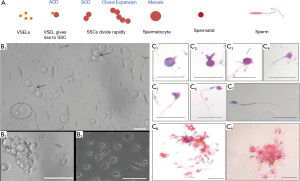
But our work was published in an Open Access journal (12) compared to Cell Stem Cell which provided a home to Zhou and co-workers’ study (1). Evidently, this difference is because they followed the gold standards proposed for in vitro derivation of germ cells (23). One needs to deliberate what was better, obtaining haploid spermatids or a large number of sperm showing all stages of stem cells biology (asymmetric cell divisions, symmetric cell divisions, clonal expansion) followed by spermatogenesis in a culture dish! The sperm generated in vitro were not tested further since we are apprehensive of sperm obtained in a dish from VSELs after 21 days culture for clinical use. We are more enthusiastic to restore spermatogenesis by endogenous manipulation of surviving stem cells in a non-functional, chemoablated gonad by injecting healthy niche cells (17) and allow Mother Nature to do the rest. Sperm collected from the caudal epithelium part of epididymis by this approach were able to fertilize eggs in vitro and initiate cleavage. Several groups have observed similar success and live births in mice on transplanting mesenchymal cells in both non-functional testis and ovary (24). A human baby has been born by transplanting autologus mesenchymal cells in a POF ovary (25).
A critique to the study (12) was that all testicular cells that survived chemotherapy were used for in vitro culture. It could possibly be the few surviving SSCs that may have given rise to sperm in vitro rather than the VSELs. Whichever the starting cells (VSELs or SSCs)—at least a huge success was demonstrated for the first time in vitro! VSELs will invariably give rise to SSCs that will further differentiate. VSELs and SSCs are not two distinct stem cell populations, rather VSELs are the stem cells and SSCs are the progenitors (26). Indeed VSELs are the ‘true’ and most primitive stem cells in the testis which give rise to SSCs by asymmetric cell division (12,22). But criticism is always good and to further satisfy the reviewers, we have now studied differentiation of mouse bone marrow derived VSELs (no chance of contaminating SSCs) on a testicular niche and observed they successfully give rise to SSCs and germ cells in vitro (personal observations).
We were intrigued to observe that PGCs were recently defined ‘unipotent’ in Nature journal (27) on the assumption that they only give rise to gametes in the body compared to totipotent or pluripotent early stage embryo/ICM which can form the whole body. This needs further clarification since like the ICM cells; even the PGCs express nuclear OCT-4 (28,29)—a transcription factor crucial to maintain pluripotent state. Loss of OCT-4 results in loss of PGCs rather than their differentiation into trophectoderm (28) suggesting an important role of nuclear OCT-4 in PGCs similar to in the ICM. In epiblast stage embryo, OCT-4 expression gets restricted to PGCs whereas the cells of all other cell lineages do no longer express OCT-4 (30,31). Similarly, conditional knockdown of Nanog (32) and SOX2 (33) also induced apoptosis and decreased numbers of migrating PGCs rather than their differentiation. Thus OCT-4, NANOG and SOX2 are crucial for PGCs; form the triumvirate to define pluripotent state (34) and their expression in PGCs reflects pluripotent state of PGCs.
VSELs are considered equivalent to PGCs and are indeed pluripotent both in situ as well as in vitro. They survive in all adult body organs (not restricted only to the gonads) and serve as a backup pool for various tissue specific stem/ progenitor cells throughout life (35,36). Also besides expressing pluripotent and PGC-specific markers, VSELs obtained from both mouse bone marrow [(37) and personal observations] and human cord blood (38) have the ability to differentiate into 3 germ layers in vitro. Scientific community needs to be convinced about the existence of VSELs and their potential. This will require revision of currently held views on basic property of PGCs (from unipotent to pluripotent) and also a re-look at the definition of the term ‘pluripotency’ (39). The deleterious effect of busulphan treatment on testicular Sertoli cells by microarray analysis and the underlying mechanism how transplantation of healthy niche (Sertoli or bone marrow mesenchymal cells) can restore spermatogenesis from endogenous VSELs in chemoablated testis was recently published (40). To conclude, endogenous VSELs are possibly better stem cell candidates to make gametes!
Acknowledgements
Financial support for the study was provided by Indian Council of Medical Research, Government of India, New Delhi, India. NIRRH Accession number IR/386/06-2016.
Footnote
Provenance: This is a Guest Commentary commissioned by Editor-in-Chief Zhizhuang Joe Zhao (Pathology Graduate Program, University of Oklahoma Health Sciences Center, Oklahoma City, USA).
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Zhou Q, Wang M, Yuan Y, et al. Complete Meiosis from Embryonic Stem Cell-Derived Germ Cells In Vitro. Cell Stem Cell 2016;18:330-40. [Crossref] [PubMed]
- Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature 1981;292:154-6. [Crossref] [PubMed]
- Irie N, Weinberger L, Tang WW, et al. SOX17 is a critical specifier of human primordial germ cell fate. Cell 2015;160:253-68. [Crossref] [PubMed]
- Hendriks S, Dancet EA, van Pelt AM, et al. Artificial gametes: a systematic review of biological progress towards clinical application. Hum Reprod Update 2015;21:285-96. [Crossref] [PubMed]
- Vassena R, Eguizabal C, Heindryckx B, et al. Stem cells in reproductive medicine: ready for the patient? Hum Reprod 2015;30:2014-21. [Crossref] [PubMed]
- Aflatoonian B, Ruban L, Jones M, et al. In vitro post-meiotic germ cell development from human embryonic stem cells. Hum Reprod 2009;24:3150-9. [Crossref] [PubMed]
- Bhartiya D. Stem cells to replace or regenerate the diabetic pancreas: Huge potential & existing hurdles. Indian J Med Res 2016;143:267-74. [Crossref] [PubMed]
- Tabar V, Studer L. Pluripotent stem cells in regenerative medicine: challenges and recent progress. Nat Rev Genet 2014;15:82-92. [Crossref] [PubMed]
- Li Y, Yang ST. Advances in human pluripotent stem cells for regenerative medicine and drug discovery. J Tissue Sci Eng 2014;5:e127.
- Zhu R, Blazeski A, Poon E, et al. Physical developmental cues for the maturation of human pluripotent stem cell-derived cardiomyocytes. Stem Cell Res Ther 2014;5:117. [Crossref] [PubMed]
- Bhartiya D, Hinduja I, Patel H, et al. Making gametes from pluripotent stem cells--a promising role for very small embryonic-like stem cells. Reprod Biol Endocrinol 2014;12:114. [Crossref] [PubMed]
- Anand S, Patel H, Bhartiya D. Chemoablated mouse seminiferous tubular cells enriched for very small embryonic-like stem cells undergo spontaneous spermatogenesis in vitro. Reprod Biol Endocrinol 2015;13:33. [Crossref] [PubMed]
- Virant-Klun I, Zech N, Rozman P, et al. Putative stem cells with an embryonic character isolated from the ovarian surface epithelium of women with no naturally present follicles and oocytes. Differentiation 2008;76:843-56. [Crossref] [PubMed]
- Parte S, Bhartiya D, Telang J, et al. Detection, characterization, and spontaneous differentiation in vitro of very small embryonic-like putative stem cells in adult mammalian ovary. Stem Cells Dev 2011;20:1451-64. [Crossref] [PubMed]
- Sriraman K, Bhartiya D, Anand S, Bhutda S. Mouse ovarian very small embryonic-like stem cells resist chemotherapy and retain ability to initiate oocyte-specific differentiation. Reprod Sci 2015;22:884-903. [Crossref] [PubMed]
- Bhartiya D, Mundekar A, Mahale V, et al. Very small embryonic-like stem cells are involved in regeneration of mouse pancreas post-pancreatectomy. Stem Cell Res Ther 2014;5:106. [Crossref] [PubMed]
- Bhartiya D. Ovarian stem cells are always accompanied by very small embryonic-like stem cells in adult mammalian ovary. J Ovarian Res 2015;8:70. [Crossref] [PubMed]
- Anand S, Bhartiya D, Sriraman K, et al. Very small embryonic-like stem cells survive and restore spermatogenesis after busulphan treatment in mouse testis. J Stem Cell Res Ther 2014;4:216.
- Bhartiya D, Kasiviswanathan S, Unni SK, et al. Newer insights into premeiotic development of germ cells in adult human testis using Oct-4 as a stem cell marker. J Histochem Cytochem 2010;58:1093-106. [Crossref] [PubMed]
- Stimpfel M, Skutella T, Kubista M, et al. Potential stemness of frozen-thawed testicular biopsies without sperm in infertile men included into the in vitro fertilization programme. J Biomed Biotechnol 2012;2012:291038.
- Kurkure P, Prasad M, Dhamankar V, et al. Very small embryonic-like stem cells (VSELs) detected in azoospermic testicular biopsies of adult survivors of childhood cancer. Reprod Biol Endocrinol 2015;13:122. [Crossref] [PubMed]
- Patel H, Bhartiya D. Testicular Stem Cells Express Follicle-Stimulating Hormone Receptors and Are Directly Modulated by FSH. Reprod Sci 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Handel MA, Eppig JJ, Schimenti JC. Applying "gold standards" to in-vitro-derived germ cells. Cell 2014;157:1257-61. [Crossref] [PubMed]
- Bhartiya D, Anand S, Parte S. VSELs may obviate cryobanking of gonadal tissue in cancer patients for fertility preservation. J Ovarian Res 2015;8:75. [Crossref] [PubMed]
- Edessy M, Hosni HN, Shady Y, et al. Autologous stem cells therapy, the first baby of idiopathic premature ovarian failure. Acta Medica International 2016;3:19-23. [Crossref]
- Bhartiya D. Stem cells, progenitors & regenerative medicine: A retrospection. Indian J Med Res 2015;141:154-61. [Crossref] [PubMed]
- Murakami K, Günesdogan U, Zylicz JJ, et al. NANOG alone induces germ cells in primed epiblast in vitro by activation of enhancers. Nature 2016;529:403-7. [Crossref] [PubMed]
- Kehler J, Tolkunova E, Koschorz B, et al. Oct4 is required for primordial germ cell survival. EMBO Rep 2004;5:1078-83. [Crossref] [PubMed]
- Pesce M, Gross MK, Schöler HR. In line with our ancestors: Oct-4 and the mammalian germ. Bioessays 1998;20:722-32. [Crossref] [PubMed]
- Schöler HR, Dressler GR, Balling R, et al. Oct-4: a germline-specific transcription factor mapping to the mouse t-complex. EMBO J 1990;9:2185-95. [PubMed]
- Schöler HR, Ruppert S, Suzuki N, et al. New type of POU domain in germ line-specific protein Oct-4. Nature 1990;344:435-9. [Crossref] [PubMed]
- Yamaguchi S, Kurimoto K, Yabuta Y, et al. Conditional knockdown of Nanog induces apoptotic cell death in mouse migrating primordial germ cells. Development 2009;136:4011-20. [Crossref] [PubMed]
- Campolo F, Gori M, Favaro R, et al. Essential role of Sox2 for the establishment and maintenance of the germ cell line. Stem Cells 2013;31:1408-21. [Crossref] [PubMed]
- Morey L, Santanach A, Di Croce L. Pluripotency and Epigenetic Factors in Mouse Embryonic Stem Cell Fate Regulation. Mol Cell Biol 2015;35:2716-28. [Crossref] [PubMed]
- Ratajczak MZ. A novel view of the adult bone marrow stem cell hierarchy and stem cell trafficking. Leukemia 2015;29:776-82. [Crossref] [PubMed]
- Bhartiya D, Shaikh A, Anand S, et al. Endogenous, very small embryonic-like stem cells: critical review, therapeutic potential and a look ahead. Hum Reprod Update 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Kucia M, Reca R, Campbell FR, et al. A population of very small embryonic-like (VSEL) CXCR4(+)SSEA-1(+)Oct-4+ stem cells identified in adult bone marrow. Leukemia 2006;20:857-69. [Crossref] [PubMed]
- Havens AM, Sun H, Shiozawa Y, et al. Human and murine very small embryonic-like cells represent multipotent tissue progenitors, in vitro and in vivo. Stem Cells Dev 2014;23:689-701. [Crossref] [PubMed]
- Bhartiya D. Intricacies of Pluripotency. J Stem Cells Regen Med 2015;11:2-6. [PubMed]
- Anand S, Bhartiya D, Sriraman K, et al. Underlying Mechanisms that Restore Spermatogenesis on Transplanting Healthy Niche Cells in Busulphan Treated Mouse Testis. Stem Cell Rev 2016. [Epub ahead of print]. [Crossref] [PubMed]
Cite this article as: Bhartiya D, Anand S, Patel H. Making gametes from pluripotent stem cells: embryonic stem cells or very small embryonic-like stem cells? Stem Cell Investig 2016;3:57.


