Friend or foe? Mogamulizumab in allogeneic hematopoietic stem cell transplantation for adult T-cell leukemia/lymphoma
Adult T-cell leukemia/lymphoma (ATL/ATLL) and mogamulizumab (moga)
ATL/ATLL is a peripheral T-cell neoplasm that occurs in around 5% of human T-lymphotropic virus type-1 (HTLV-1) carriers and is one of the most aggressive hematologic malignancies (1,2). HTLV-1 is endemic in some areas, such as coastal regions of southwest Japan, South America, and Africa (2). ATL comprises four clinical subtypes, namely, acute, lymphoma, chronic, and smoldering, the former two of which show more aggressive courses (2). Generally, ATL responds to primary chemotherapy, but it soon becomes refractory to multiple anticancer reagents (3). Therefore, even the currently most intensive chemotherapy regimen modified LSG15 (mLSG15, VCAP-AMP-VECP) results in a median survival of only 13 months (4). Although allogeneic hematopoietic stem cell transplantation (allo-HSCT) may lead to long-term remission in a proportion of patients with aggressive ATL (5), it can also cause fatal complications such as graft-versus-host disease (GVHD) (6).
Interestingly, there are several lines of evidence that tumor immunity can suppress ATL. Some cases experience spontaneous remission without any anticancer treatment (7-10). The occurrence of mild GVHD correlates with reduced relapse of ATL patients after allo-HSCT (11). Chemotherapy-resistant cases with substantial tumor burdens sometimes achieve long-term remission after allo-HSCT (12). Even relapsing cases after allo-HSCT still respond and achieve complete remission again only following withdrawal of immunosuppressants (13). These observations may be due to the high immunogenicity of ATL cells such as the tumor antigens Tax or NY-ESO-1, and several projects have aimed to treat ATL by enhancing tumor immunity (14,15).
The advent of clinically feasible monoclonal antibodies enabled specific depletion of tumor cells. They enhance antitumor immunity by antibody-dependent cellular cytotoxicity (ADCC) or complement-dependent cytotoxicity (CDC) (16). As for hematologic malignancies, the anti-CD20 antibody rituximab gained FDA approval for B cell-lineage malignant lymphoma in 1997. Since then, many antibodies have been developed, and their specificities and potentials have been greatly improved. Currently, novel monoclonal antibodies that target immune checkpoint signals such as nivolumab and ipilimumab are about to change cancer therapy (17,18). However, their adverse effects can result in unknown severe pathology, partly due to incomplete understanding of the human immune system. Therefore, careful and close monitoring of cases that are treated with immunomodulatory reagents is needed.
Chemokines and their receptors play important roles in tumorigenesis and the expansion and migration potentials of tumor cells in the body (19). Moga, a monoclonal antibody that targets the chemokine receptor CCR4, was developed in Japan (20,21). Many T-cell neoplasms including ATL express CCR4 (22,23), for which moga gained approval for clinical use. It binds to CCR4 on ATL cells, inducing ADCC by natural killer (NK) cells (Figure 1). The most notable feature of moga is elimination of fucose from sugar chains on the antibody by Potelligent® technology, which strikingly enhances its ADCC activity (24).
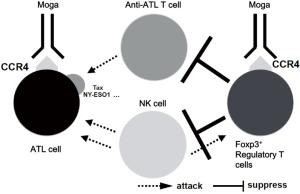
Moga shows substantial antitumor activity against relapsed/refractory ATL and peripheral T-cell lymphoma. Notably, it is effective even in chemotherapy-resistant or relapsing cases, which means that many unfavorable ATL cases can achieve remission using moga (25,26). Furthermore, its indication may be widened to solid organ malignancies (27). However, there are still some problems to be solved or overcome. Although the effects of moga are often dramatic, some ATL cases are still resistant. There is no clear conclusion regarding the appropriate combination therapy of moga with conventional chemotherapy (28). The effects of moga on extramedullary lesions are limited (25,29). Furthermore, moga has immunomodulatory effects, including depleting regulatory T cells (Tregs) (30). Therefore, concerns have been raised regarding the use of moga, especially prior to allo-HSCT. In this review, the risks of the use of moga in transplant settings and some possible approaches to avoid adverse events are discussed.
Moga and Tregs
Foxp3+ Tregs are an indispensable cell subset in human immunity. Because they suppress antitumor immunity in addition to autoimmunity, suppression of Tregs enhances antitumor immunity. Depletion of Tregs reduces the tumor burden in vivo in mice (31,32). In humans, the higher density of Tregs among tumor-infiltrating lymphocytes is associated with poor prognosis in several cancers (33-36). Tregs are classified into several subtypes: the most suppressive subset has the CD45RA−Foxp3++ phenotype, called effector Tregs (37). Effective depletion of effector Tregs may be crucial to achieve strong antitumor immunity (30). It should be noted that effector Tregs express CCR4 (30) and that depletion of Tregs might mount autoimmune pathology.
Tregs are also important in allo-HSCT. They appropriately modulate immunity, establish graft tolerance, enhance engraftment, and suppress GVHD (38,39). A reduced frequency of Tregs correlates with chronic GVHD (40). Although a reduction in Tregs should mount substantial antitumor immunity, depletion of Tregs may increase severe complications such as GVHD in allo-HSCT.
Several reports have indicated that ATL cells and Tregs share similar features, such as the CD3+CD4+CD25+ phenotype (41). Although they can be differentiated by CADM1 antigen expression (41), they share the CCR4+Foxp3+ phenotype in many cases. Therefore, moga might deplete Tregs in addition to ATL cells. Moga results in severe autoimmune pathology coincident with depletion of Tregs (41). In addition, T cells with the Th2 phenotype also express CCR4 (42). Theoretically, moga would shift the Th1/Th2 balance to the Th1 axis, which might enhance tissue damage through GVHD, although this has not been sufficiently investigated yet.
Collectively, while moga should enhance antitumor immunity, it may be problematic in cases that subsequently receive allo-HSCT because it can increase the risks of GVHD, graft rejection, impaired immune reconstitution, and other post-transplant complications.
Moga and establishment of tolerance after allo-HSCT
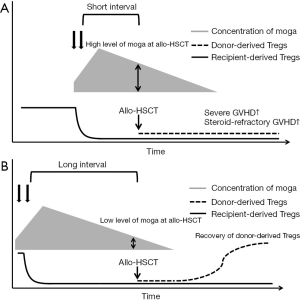
As described above, there are major concerns that pretransplant moga could increase the risk of GVHD. Recently, several groups reported the clinical outcomes of cases that received moga before allo-HSCT. These studies consistently reported that the use of moga before allo-HSCT was associated with an increased risk of severe acute GVHD (43-46), although a case report showed the successful management of acute GVHD (45). However, these studies were rather small to conduct multivariate analyses to adjust for the other risk factors of acute GVHD and other clinical events. Our group recently performed a retrospective analysis using a database of a nationwide survey of aggressive ATL (12). In this study, 82 patients out of 996 allo-HSCT recipients received moga before allo-HSCT. The risk of grade III–IV acute GVHD and steroid-refractory acute GVHD was significantly higher in patients who received moga before allo-HSCT than in those who did not receive moga before allo-HSCT. The cumulative incidence of non-relapse mortality was significantly higher in the moga group than in the no-moga group (43.7% in the moga group and 25.1% in the no-moga group at 1 year). There was no significant difference in the incidence of relapse between the two groups. As a result, the probability of overall survival in the moga group was significantly inferior to that in the no-moga group (32.2% in the moga group and 49.4% in the no-moga group at 1 year). The median interval between the last moga administration and allo-HSCT was 45 days in this study. Using 50 days as a cut-off, a shorter interval between the last moga administration and allo-HSCT was significantly associated with an increased risk of non-relapse mortality and overall mortality. Sugio et al. suggested that an interval of <3 months between the last moga administration and allo-HSCT might be associated with an inferior clinical outcome (46). According to some previous reports, the concentration of moga remains more than 10 µg/mL for weeks even after the last administration of 1.0 mg/kg (25). Although we do not have any data about the correlation of immune recovery and plasma concentrations of moga, we assume that in patients with a shorter interval between the last moga administration and allo-HSCT, the concentration of moga remained high enough to deplete donor-derived Tregs after allo-HSCT, which is expected to induce severe acute GVHD as shown in Figure 2. In patients with longer intervals between the last moga administration and allo-HSCT, moga would deplete recipient-derived Tregs, but might not be able to deplete donor-derived Tregs. Recent reports showed that the majority of Tregs in the early period after transplant shows a CD45RA− effector/memory phenotype (30,47). Because effector/memory phenotype Tregs express CCR4, the use of moga might result in a critical decrease in the overall Treg population during the early period after allo-HSCT (47,48). To elucidate the mechanisms in vivo further, prospective monitoring of the moga level and immune recovery including Tregs in peripheral blood is warranted.
How to incorporate moga in transplant-eligible patients with ATL
Considering the dismal outcome after allo-HSCT in patients who received moga before allo-HSCT, moga should be cautiously used in transplant-eligible patients with ATL. However, as described above, a proportion of patients with relapsed/refractory ATL could be rescued by moga (25,26). Therefore, incorporation of moga into the salvage treatment strategy might increase the number of potential candidates for allo-HSCT, and the development of transplant methods to maximize the benefits of moga in the treatment strategy of aggressive ATL is desired.
First, we can prolong the interval between the last moga administration and allo-HSCT. As previously reported and theoretically, the effects of moga could be reduced because the concentration of moga could be lowered as shown in Figure 2. Therefore, we could use moga in combination with other chemotherapies or moga alone in patients with relapsed/refractory ATL but only use moga for a short period and continue conventional salvage chemotherapy after sufficient disease control is achieved. The drawback of this strategy is that there is a possibility that disease control could worsen after moga is stopped and that the appropriate interval may depend on each patient. In our experience, this strategy is highly efficient in ATL cases with tumor cells only in peripheral blood (25,26). As aforementioned, moga is expected to persist for several months in vivo as the half-life of moga is approximately 16 to 18 days (26). In addition, the effects of moga may last for more, because its pharmacodynamics may be different from its pharmacokinetics. Thus, we have to pay much careful attention to the development of severe GVHD, even when a long interval exists between administration of moga and allo-HSCT.
Second, we can intensify GVHD prophylaxis in patients who received moga before allo-HSCT. It is expected that a higher ratio of effector T cells/Tregs might lead to the development of acute GVHD (49). Although there are no data regarding how to intensify GVHD prophylaxis in patients who received moga, we could incorporate anti-thymocyte globulin (ATG) to deplete effector T cells and induce Tregs. ATG was reported to deplete effector T cells but possibly expand Tregs (50-54). The dose of ATG that is practically used differs among centers/countries (55-57). A low dose of ATG (58-61) is usually used in Japan because previous reports showed that the incidence of acute GVHD is lower in the Japanese population than in the Caucasian population (62-64). However, in patients who received moga, it is expected that higher doses of ATG are needed, which should be determined in the future. Although post-transplant cyclophosphamide (PT-Cy) might be an option as a potent GVHD prophylaxis, GVHD prophylaxis using PT-CY relies heavily on the expansion of Tregs (65,66) and seems ineffective in patients who received moga before allo-HSCT in our experience (personal communication).
Third, we can possibly use adoptive Treg therapy, although it is not usually available in clinical practice. Several studies showed promising results using adoptive Tregs (67-74). However, before adoptive Treg therapy is a cellular therapeutic agent, there are various hurdles to overcome (75). We need to identify and isolate Tregs, and expand them under good manufacturing practice for cellular product manufacturing.
In addition, it is important that some patients do not experience any immunological complications even after a short interval of moga use. This diversity might depend on some polymorphisms such as HLA and KIR, which should be further investigated.
On the other hand, moga might be incorporated as consolidation or salvage therapy “after” allogeneic HSCT. Ipilimumab, a checkpoint inhibitor, was reported to have some beneficial effects in relapses after allogeneic HSCT despite the risk of GVHD (76). Although there have not been sufficient data about the feasibility and efficacy of posttransplant moga, moga might be safely administered as ipilimumab. In any case, however, we should carefully monitor the incidence of adverse events, as moga could induce GVHD and other alloreaction-related complications.
In summary, because moga might have beneficial effects in a significant proportion of patients, we need to conduct prospective studies to establish a way to inhibit severe/steroid-refractory GVHD in patients who have received pretransplant moga.
Conclusions
Moga has a therapeutic potential in patients with relapsed/refractory ATL. To improve the clinical outcome of relapsed/refractory ATL, we need to develop a treatment strategy incorporating chemotherapy, moga, and allo-HSCT. To optimize such a treatment strategy, more studies are needed to clarify the effects of moga on immune tolerance and tumor immunity.
Acknowledgements
None.
Footnote
Conflict of Interest: The authors have no conflicts of interest to declare.
References
- Uchiyama T, Yodoi J, Sagawa K, et al. Adult T-cell leukemia: clinical and hematologic features of 16 cases. Blood 1977;50:481-92. [PubMed]
- Ishitsuka K, Tamura K. Human T-cell leukaemia virus type I and adult T-cell leukaemia-lymphoma. Lancet Oncol 2014;15:e517-26. [Crossref] [PubMed]
- Fukushima T, Nomura S, Shimoyama M, et al. Japan Clinical Oncology Group (JCOG) prognostic index and characterization of long-term survivors of aggressive adult T-cell leukaemia-lymphoma (JCOG0902A). Br J Haematol 2014;166:739-48. [Crossref] [PubMed]
- Tsukasaki K, Utsunomiya A, Fukuda H, et al. VCAP-AMP-VECP compared with biweekly CHOP for adult T-cell leukemia-lymphoma: Japan Clinical Oncology Group Study JCOG9801. J Clin Oncol 2007;25:5458-64. [Crossref] [PubMed]
- Utsunomiya A, Miyazaki Y, Takatsuka Y, et al. Improved outcome of adult T cell leukemia/lymphoma with allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant 2001;27:15-20. [Crossref] [PubMed]
- Ishida T, Hishizawa M, Kato K, et al. Allogeneic hematopoietic stem cell transplantation for adult T-cell leukemia-lymphoma with special emphasis on preconditioning regimen: a nationwide retrospective study. Blood 2012;120:1734-41. [Crossref] [PubMed]
- Murakawa M, Shibuya T, Teshima T, et al. Spontaneous remission from acute exacerbation of chronic adult T-cell leukemia. Blut 1990;61:346-9. [Crossref] [PubMed]
- Schnitzer B, Lovett EJ 3rd, Kahn LE. Adult T-cell leukaemia with spontaneous remission. Lancet 1983;2:1030. [Crossref] [PubMed]
- Takezako Y, Kanda Y, Arai C, et al. Spontaneous remission in acute type adult T-cell leukemia/lymphoma. Leuk Lymphoma 2000;39:217-22. [Crossref] [PubMed]
- Taniguchi S, Yamasaki K, Shibuya T, et al. Spontaneous remission of acute adult T-cell leukaemia with chromosomal abnormality infiltrating to skin and liver. Br J Haematol 1993;85:413-4. [Crossref] [PubMed]
- Kanda J, Hishizawa M, Utsunomiya A, et al. Impact of graft-versus-host disease on outcomes after allogeneic hematopoietic cell transplantation for adult T-cell leukemia: a retrospective cohort study. Blood 2012;119:2141-8. [Crossref] [PubMed]
- Fuji S, Inoue Y, Utsunomiya A, et al. Pretransplant anti-CCR4 antibody mogamulizumab against ATLL is associated with significantly increased risks of severe and steroid-refractory GVHD, non-relapse mortality and overall mortality. J Clin Oncol 2016;34:3426-33. [Crossref] [PubMed]
- Yonekura K, Utsunomiya A, Takatsuka Y, et al. Graft-versus-adult T-cell leukemia/lymphoma effect following allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant 2008;41:1029-35. [Crossref] [PubMed]
- Nishikawa H, Maeda Y, Ishida T, et al. Cancer/testis antigens are novel targets of immunotherapy for adult T-cell leukemia/lymphoma. Blood 2012;119:3097-104. [Crossref] [PubMed]
- Suehiro Y, Hasegawa A, Iino T, et al. Clinical outcomes of a novel therapeutic vaccine with Tax peptide-pulsed dendritic cells for adult T cell leukaemia/lymphoma in a pilot study. Br J Haematol 2015;169:356-67. [Crossref] [PubMed]
- Scott AM, Wolchok JD, Old LJ. Antibody therapy of cancer. Nat Rev Cancer 2012;12:278-87. [Crossref] [PubMed]
- Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012;366:2455-65. [Crossref] [PubMed]
- Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366:2443-54. [Crossref] [PubMed]
- Vela M, Aris M, Llorente M, et al. Chemokine receptor-specific antibodies in cancer immunotherapy: achievements and challenges. Front Immunol 2015;6:12. [Crossref] [PubMed]
- Shinkawa T, Nakamura K, Yamane N, et al. The absence of fucose but not the presence of galactose or bisecting N-acetylglucosamine of human IgG1 complex-type oligosaccharides shows the critical role of enhancing antibody-dependent cellular cytotoxicity. J Biol Chem 2003;278:3466-73. [Crossref] [PubMed]
- Ito A, Ishida T, Yano H, et al. Defucosylated anti-CCR4 monoclonal antibody exercises potent ADCC-mediated antitumor effect in the novel tumor-bearing humanized NOD/Shi-scid, IL-2Rgamma(null) mouse model. Cancer Immunol Immunother 2009;58:1195-206. [Crossref] [PubMed]
- Yoshie O, Fujisawa R, Nakayama T, et al. Frequent expression of CCR4 in adult T-cell leukemia and human T-cell leukemia virus type 1-transformed T cells. Blood 2002;99:1505-11. [Crossref] [PubMed]
- Ishida T, Inagaki H, Utsunomiya A, et al. CXC chemokine receptor 3 and CC chemokine receptor 4 expression in T-cell and NK-cell lymphomas with special reference to clinicopathological significance for peripheral T-cell lymphoma, unspecified. Clin Cancer Res 2004;10:5494-500. [Crossref] [PubMed]
- Yano H, Ishida T, Imada K, et al. Augmentation of antitumour activity of defucosylated chimeric anti-CCR4 monoclonal antibody in SCID mouse model of adult T-cell leukaemia/lymphoma using G-CSF. Br J Haematol 2008;140:586-9. [Crossref] [PubMed]
- Ishida T, Joh T, Uike N, et al. Defucosylated anti-CCR4 monoclonal antibody (KW-0761) for relapsed adult T-cell leukemia-lymphoma: a multicenter phase II study. J Clin Oncol 2012;30:837-42. [Crossref] [PubMed]
- Yamamoto K, Utsunomiya A, Tobinai K, et al. Phase I study of KW-0761, a defucosylated humanized anti-CCR4 antibody, in relapsed patients with adult T-cell leukemia-lymphoma and peripheral T-cell lymphoma. J Clin Oncol 2010;28:1591-8. [Crossref] [PubMed]
- Kurose K, Ohue Y, Wada H, et al. Phase Ia Study of FoxP3+ CD4 Treg Depletion by Infusion of a Humanized Anti-CCR4 Antibody, KW-0761, in Cancer Patients. Clin Cancer Res 2015;21:4327-36. [Crossref] [PubMed]
- Ishida T, Jo T, Takemoto S, et al. Dose-intensified chemotherapy alone or in combination with mogamulizumab in newly diagnosed aggressive adult T-cell leukaemia-lymphoma: a randomized phase II study. Br J Haematol 2015;169:672-82. [Crossref] [PubMed]
- Tsutsumi Y, Shimono J, Miyashita N, et al. No effect of humanized CCR monoclonal antibody (mogamulizumab) on treatment-resistant adult T cell leukemia with meningeal infiltration. Leuk Lymphoma 2014;55:457-9. [Crossref] [PubMed]
- Sugiyama D, Nishikawa H, Maeda Y, et al. Anti-CCR4 mAb selectively depletes effector-type FoxP3+CD4+ regulatory T cells, evoking antitumor immune responses in humans. Proc Natl Acad Sci U S A 2013;110:17945-50. [Crossref] [PubMed]
- Onizuka S, Tawara I, Shimizu J, et al. Tumor rejection by in vivo administration of anti-CD25 (interleukin-2 receptor alpha) monoclonal antibody. Cancer Res 1999;59:3128-33. [PubMed]
- Shimizu J, Yamazaki S, Sakaguchi S. Induction of tumor immunity by removing CD25+CD4+ T cells: a common basis between tumor immunity and autoimmunity. J Immunol 1999;163:5211-8. [PubMed]
- Curiel TJ, Coukos G, Zou L, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med 2004;10:942-9. [Crossref] [PubMed]
- Sato E, Olson SH, Ahn J, et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci U S A 2005;102:18538-43. [Crossref] [PubMed]
- Badoual C, Hans S, Rodriguez J, et al. Prognostic value of tumor-infiltrating CD4+ T-cell subpopulations in head and neck cancers. Clin Cancer Res 2006;12:465-72. [Crossref] [PubMed]
- Bates GJ, Fox SB, Han C, et al. Quantification of regulatory T cells enables the identification of high-risk breast cancer patients and those at risk of late relapse. J Clin Oncol 2006;24:5373-80. [Crossref] [PubMed]
- Miyara M, Yoshioka Y, Kitoh A, et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity 2009;30:899-911. [Crossref] [PubMed]
- Hoffmann P, Ermann J, Edinger M, et al. Donor-type CD4(+)CD25(+) regulatory T cells suppress lethal acute graft-versus-host disease after allogeneic bone marrow transplantation. J Exp Med 2002;196:389-99. [Crossref] [PubMed]
- Taylor PA, Noelle RJ, Blazar BR. CD4(+)CD25(+) immune regulatory cells are required for induction of tolerance to alloantigen via costimulatory blockade. J Exp Med 2001;193:1311-8. [Crossref] [PubMed]
- Zorn E, Kim HT, Lee SJ, et al. Reduced frequency of FOXP3+ CD4+CD25+ regulatory T cells in patients with chronic graft-versus-host disease. Blood 2005;106:2903-11. [Crossref] [PubMed]
- Ureshino H, Shindo T, Nishikawa H, et al. Effector Regulatory T Cells Reflect the Equilibrium between Antitumor Immunity and Autoimmunity in Adult T-cell Leukemia. Cancer Immunol Res 2016;4:644-9. [Crossref] [PubMed]
- Imai T, Nagira M, Takagi S, et al. Selective recruitment of CCR4-bearing Th2 cells toward antigen-presenting cells by the CC chemokines thymus and activation-regulated chemokine and macrophage-derived chemokine. Int Immunol 1999;11:81-8. [Crossref] [PubMed]
- Haji S, Kiyasu J, Choi I, et al. Administration of an anti-CC chemokine receptor 4 monoclonal antibody, mogamulizumab, before allogeneic bone marrow transplantation for adult T-cell leukemia/lymphoma. Bone Marrow Transplant 2016;51:432-4. [Crossref] [PubMed]
- Inoue Y, Fuji S, Tanosaki R, et al. Pretransplant mogamulizumab against ATLL might increase the risk of acute GVHD and non-relapse mortality. Bone Marrow Transplant 2016;51:725-7. [Crossref] [PubMed]
- Motohashi K, Suzuki T, Kishimoto K, et al. Successful treatment of a patient with adult T cell leukemia/lymphoma using anti-CC chemokine receptor 4 monoclonal antibody mogamulizumab followed by allogeneic hematopoietic stem cell transplantation. Int J Hematol 2013;98:258-60. [Crossref] [PubMed]
- Sugio T, Kato K, Aoki T, et al. Mogamulizumab Treatment Prior to Allogeneic Hematopoietic Stem Cell Transplantation Induces Severe Acute Graft-versus-Host Disease. Biol Blood Marrow Transplant 2016;22:1608-14. [Crossref] [PubMed]
- Matsuoka K, Kim HT, McDonough S, et al. Altered regulatory T cell homeostasis in patients with CD4+ lymphopenia following allogeneic hematopoietic stem cell transplantation. J Clin Invest 2010;120:1479-93. [Crossref] [PubMed]
- Inoue T, Ikegame K, Kaida K, et al. Host Foxp3+CD4+ Regulatory T Cells Act as a Negative Regulator of Dendritic Cells in the Peritransplantation Period. J Immunol 2016;196:469-83. [Crossref] [PubMed]
- Alho AC, Kim HT, Chammas MJ, et al. Unbalanced recovery of regulatory and effector T cells after allogeneic stem cell transplantation contributes to chronic GVHD. Blood 2016;127:646-57. [Crossref] [PubMed]
- Feng X, Kajigaya S, Solomou EE, et al. Rabbit ATG but not horse ATG promotes expansion of functional CD4+CD25highFOXP3+ regulatory T cells in vitro. Blood 2008;111:3675-83. [Crossref] [PubMed]
- Sewgobind VD, van der Laan LJ, Kho MM, et al. The calcineurin inhibitor tacrolimus allows the induction of functional CD4CD25 regulatory T cells by rabbit anti-thymocyte globulins. Clin Exp Immunol 2010;161:364-77. [PubMed]
- Lopez M, Clarkson MR, Albin M, et al. A novel mechanism of action for anti-thymocyte globulin: induction of CD4+CD25+Foxp3+ regulatory T cells. J Am Soc Nephrol 2006;17:2844-53. [Crossref] [PubMed]
- Shimony O, Nagler A, Gellman YN, et al. Anti-T lymphocyte globulin (ATG) induces generation of regulatory T cells, at least part of them express activated CD44. J Clin Immunol 2012;32:173-88. [Crossref] [PubMed]
- Ruzek MC, Waire JS, Hopkins D, et al. Characterization of in vitro antimurine thymocyte globulin-induced regulatory T cells that inhibit graft-versus-host disease in vivo. Blood 2008;111:1726-34. [Crossref] [PubMed]
- Binkert L, Medinger M, Halter JP, et al. Lower dose anti-thymocyte globulin for GvHD prophylaxis results in improved survival after allogeneic stem cell transplantation. Bone Marrow Transplant 2015;50:1331-6. [Crossref] [PubMed]
- Kröger N, Solano C, Wolschke C, et al. Antilymphocyte Globulin for Prevention of Chronic Graft-versus-Host Disease. N Engl J Med 2016;374:43-53. [Crossref] [PubMed]
- Walker I, Panzarella T, Couban S, et al. Pretreatment with anti-thymocyte globulin versus no anti-thymocyte globulin in patients with haematological malignancies undergoing haemopoietic cell transplantation from unrelated donors: a randomised, controlled, open-label, phase 3, multicentre trial. Lancet Oncol 2016;17:164-73. [Crossref] [PubMed]
- Fuji S, Kim SW, Yano S, et al. A prospective multicenter study of unrelated bone marrow transplants using a reduced-intensity conditioning regimen with low-dose ATG-F. Bone Marrow Transplant 2016;51:451-3. [Crossref] [PubMed]
- Fuji S, Ueno N, Hiramoto N, et al. Reduced-intensity conditioning regimen with low-dose ATG-F for unrelated bone marrow transplant is associated with lower non-relapse mortality than a regimen with low-dose TBI: a single-center retrospective analysis of 103 cases. Int J Hematol 2013;98:608-14. [Crossref] [PubMed]
- Hatanaka K, Fuji S, Ikegame K, et al. Low incidences of acute and chronic graft-versus-host disease after unrelated bone marrow transplantation with low-dose anti-T lymphocyte globulin. Int J Hematol 2012;96:773-80. [Crossref] [PubMed]
- Kuriyama K, Fuji S, Inamoto Y, et al. Impact of low-dose rabbit anti-thymocyte globulin in unrelated hematopoietic stem cell transplantation. Int J Hematol 2016;103:453-60. [Crossref] [PubMed]
- Oh H, Loberiza FR Jr, Zhang MJ, et al. Comparison of graft-versus-host-disease and survival after HLA-identical sibling bone marrow transplantation in ethnic populations. Blood 2005;105:1408-16. [Crossref] [PubMed]
- Hahn T, McCarthy PL Jr, Zhang MJ, et al. Risk factors for acute graft-versus-host disease after human leukocyte antigen-identical sibling transplants for adults with leukemia. J Clin Oncol 2008;26:5728-34. [Crossref] [PubMed]
- Morishima Y, Kawase T, Malkki M, et al. Significance of ethnicity in the risk of acute graft-versus-host disease and leukemia relapse after unrelated donor hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2013;19:1197-203. [Crossref] [PubMed]
- Ganguly S, Ross DB, Panoskaltsis-Mortari A, et al. Donor CD4+ Foxp3+ regulatory T cells are necessary for posttransplantation cyclophosphamide-mediated protection against GVHD in mice. Blood 2014;124:2131-41. [Crossref] [PubMed]
- Kanakry CG, Ganguly S, Zahurak M, et al. Aldehyde dehydrogenase expression drives human regulatory T cell resistance to posttransplantation cyclophosphamide. Sci Transl Med 2013;5:211ra157. [Crossref] [PubMed]
- Taylor PA, Lees CJ, Blazar BR. The infusion of ex vivo activated and expanded CD4(+)CD25(+) immune regulatory cells inhibits graft-versus-host disease lethality. Blood 2002;99:3493-9. [Crossref] [PubMed]
- Hoffmann P, Eder R, Kunz-Schughart LA, et al. Large-scale in vitro expansion of polyclonal human CD4(+)CD25high regulatory T cells. Blood 2004;104:895-903. [Crossref] [PubMed]
- Brunstein CG, Miller JS, Cao Q, et al. Infusion of ex vivo expanded T regulatory cells in adults transplanted with umbilical cord blood: safety profile and detection kinetics. Blood 2011;117:1061-70. [Crossref] [PubMed]
- Trzonkowski P, Bieniaszewska M, Juścińska J, et al. First-in-man clinical results of the treatment of patients with graft versus host disease with human ex vivo expanded CD4+CD25+CD127- T regulatory cells. Clin Immunol 2009;133:22-6. [Crossref] [PubMed]
- Martelli MF, Di Ianni M, Ruggeri L, et al. HLA-haploidentical transplantation with regulatory and conventional T-cell adoptive immunotherapy prevents acute leukemia relapse. Blood 2014;124:638-44. [Crossref] [PubMed]
- Di Ianni M, Falzetti F, Carotti A, et al. Tregs prevent GVHD and promote immune reconstitution in HLA-haploidentical transplantation. Blood 2011;117:3921-8. [Crossref] [PubMed]
- Bacchetta R, Lucarelli B, Sartirana C, et al. Immunological Outcome in Haploidentical-HSC Transplanted Patients Treated with IL-10-Anergized Donor T Cells. Front Immunol 2014;5:16. [Crossref] [PubMed]
- Theil A, Tuve S, Oelschlägel U, et al. Adoptive transfer of allogeneic regulatory T cells into patients with chronic graft-versus-host disease. Cytotherapy 2015;17:473-86. [Crossref] [PubMed]
- Trzonkowski P, Bacchetta R, Battaglia M, et al. Hurdles in therapy with regulatory T cells. Sci Transl Med 2015;7:304ps18. [Crossref] [PubMed]
- Davids MS, Kim HT, Bachireddy P, et al. Ipilimumab for Patients with Relapse after Allogeneic Transplantation. N Engl J Med 2016;375:143-53. [Crossref] [PubMed]
Cite this article as: Fuji S, Shindo T. Friend or foe? Mogamulizumab in allogeneic hematopoietic stem cell transplantation for adult T-cell leukemia/lymphoma. Stem Cell Investig 2016;3:70.


