Novel direct reprogramming technique for the generation of culture-expandable cardiac progenitor cells from fibroblasts
Since the tremendous discovery of induce-pluripotent stem cells (iPSCs) in 2006 (1,2), investigators have been trying to induce somatic cell reprogramming into certain lineage cells following the basic method, in which lineage-related critical genes are initially screened out of numerous candidates and several master genes are then selected by transduction of multiple genes with appropriate culture conditions in somatic cells confirming transdifferentiation of the transduced cells. The technique is called as “direct reprogramming” which can avoid generating iPSCs and previous studies have successfully demonstrated lineage reprogramming into a variety of differentiated cell types such as neuronal cells (3), hepatocytes (4), and cardiomyocytes (5). Despite of the achievement of direct lineage reprogramming, investigators next challenged the generation of progenitor cells rather than terminally differentiated cells by direct reprogramming, because progenitor cells are generally proliferative and have advantage of large scale expansion as a tool for regeneration therapy. Indeed, recent reports exhibited that reprogrammed progenitors of neuronal cells (3), hepatocytes (6), and cardiomyocytes (7) were capable of proliferation.
Cardiac progenitor cells (CPCs) have been identified with a variety of markers (Table 1) including: (I) transcription factors of Mesp1, Ist1, and Nkx2.5 in embryonic stem cells, (II) cell surface proteins of CXCR4, PDGFR-a, Flk-1/KDR, and SIRPA in pluripotent stem cells, and (III) cell surface proteins of Sca-1 and c-Kit in mammalian somatic stem cells. On the other hand, induced-CPCs including cardiomyocyte-like cells have also been attempted to generate from fibroblasts by direct reprogramming methods (Table 2). In the very recent study, Lalit et al. (7) reported that a combination of cardiac factors and signaling molecules reprogramed adult mouse fibroblasts into expandable induced cardiac progenitor cells (iCPCs). The iCPCs were multipotent and could differentiate into not only cardiomyocytes but also smooth muscle cells and endothelial cells that also could be sources of myocardium. Moreover, iCPCs generate myocardium when injected into the embryonic and adult post-MI mouse heart.
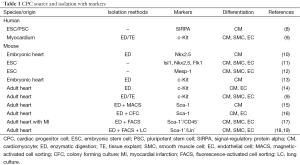
Full table
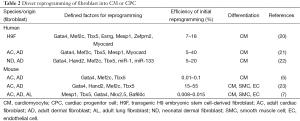
Full table
First, the authors focused on 22 genes including 18 candidate genes that are critical during embryonic cardiovascular development and 4 iPSC factors, and finally selected 5 genes (Gata4, Mesp1, Tbx5, Nkx2.5, and Baf60c) for generating CPCs utilizing an Nkx2.5 cardiac reporter mouse model expressing enhanced yellow fluorescent protein (EYFP). The source of fibroblasts was chosen from adult but not neonatal fibroblasts for reprogramming in terms of clinical applicability. They next worked out optimizing culture conditions to let the generated CPCs to proliferate/expand with activating Wnt and JAK/STAT signaling by chemical compounds. Intriguingly, the proliferating iCPCs exhibited neither protein expression for pluripotency of Oct4 nor cardiovascular lineage differentiation markers of α-actinin, smooth muscle-myosin heavy chain, and CD31 even after extensive passaging. It was suggested that the genetically engineered iCPCs under specific culture conditions could be expanded keeping their characteristics as progenitor cells. iCPCs were successfully induced from not only cardiac fibroblasts but also adult lung and adult tail tip (dermal) fibroblasts.
For iCPC differentiation into cardiovascular lineages, iCPCs exited progenitor state losing Nkx2.5 gene expression and differentiated into CM marker-positive cells including MLC-2v-/MLC-2a-positive ventricle-/atrium-like cells (80–90%), SMC marker-positive cells (5–10%), and EC marker-positive cells (1–5%) over 20 days after plating with cardiac differentiation medium in culture. Although the iCPC-derived CMs were not spontaneously contracting, co-culture with ES-derived CMs allowed them contract expressing calcium transients. These findings indicate that the iCPCs spontaneously differentiated into mainly cardiomyocytes rather than vascular cells under cardiac differentiation condition, however, culture under vascular differentiation conditions may increase the differentiation frequency into SMCs and ECs in iCPCs. When the iCPCs were transplanted in the cardiac crescent of mouse embryos, iCPC-derived cells integrated with host cells within the heart tube and iCPC-derived CMs were observed in developing both atria and ventricles as well as outflow track, demonstrating no spatial preference within the heart tube. In contrast, iCPC-derived CD31 positive ECs or SMCs failed to detect in vivo even though endothelial and smooth muscle differentiation from iCPCs could be observed in vitro. This discrepancy might be due to the complexity of cardiac developmental regulations in embryos. Finally, the iCPCs were transplanted in adult mouse heart after myocardial infarction (MI) to examine if the transplanted iCPCs differentiate into cardiovascular cells in ischemic myocardium. One and half million of iCPCs were injected to ischemic myocardium (ischemic border zone) 2 days following MI induction. Some of the injected iCPCs could be detected as cardiovascular cell marker-positive cells in histological sections of ischemic myocardium 28 days after cell injection exhibiting significantly increased survival rate compared with the control. Although the remained iCPCs are thought to be differentiated into cardiovascular cells, thinking about 1.0–1.5×106 of iCPC cardiac injection, the remained cell number would not be enough to contribute to improve cardiac function. The favorable effect of the large number of cardiac iCPC injection appears to be due to the indirect/paracrine effect, i.e., secreted growth factor/cytokine/anti-apoptosis factor, rather than direct contribution to tissue regeneration. Also, if the iCPCs have a homing capacity to ischemic myocardium, the intravenous drip infusion or direct infusion via coronary arteries of iCPCs but not cardiac local injection with open chest surgery would be less invasive and easy/useful way of cell transplantation as a treatment.
In conclusion, the present study demonstrated that CPCs were successfully induced from murine fibroblasts derived from 3 different organ sources by direct reprogramming method with new 5 gene combinations and JAK/STAT signaling activation. In terms of clinical application for cardiovascular diseases, the iCPCs have advantages because of its expansion capacity in culture compared with the previously reported differentiated reprogrammed cardiomyocytes or cardiomyocyte-like cells. The next issue to be tested would be the generation of directly reprogrammed iCPCs from human fibroblasts or other cell types, i.e., lymphocyte which is easy to harvest from peripheral blood avoiding abnormal karyotype after genetic modifications. Ideally speaking, higher frequency of the transplanted iCPC recruitment/differentiation into cardiovascular lineages in postnatal ischemic myocardium will be required for practical cardiovascular regeneration therapy. Nevertheless, this study gave rise to a great progress in the research filed of direct reprogramming with somatic cells for the generation of cardiovascular cells.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006;126:663-76. [Crossref] [PubMed]
- Yu J, Vodyanik MA, Smuga-Otto K, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 2007;318:1917-20. [Crossref] [PubMed]
- Han DW, Tapia N, Hermann A, et al. Direct reprogramming of fibroblasts into neural stem cells by defined factors. Cell Stem Cell 2012;10:465-72. [Crossref] [PubMed]
- Sekiya S, Suzuki A. Direct conversion of mouse fibroblasts to hepatocyte-like cells by defined factors. Nature 2011;475:390-3. [Crossref] [PubMed]
- Ieda M, Fu JD, Delgado-Olguin P, et al. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell 2010;142:375-86. [Crossref] [PubMed]
- Yu B, He ZY, You P, et al. Reprogramming fibroblasts into bipotential hepatic stem cells by defined factors. Cell Stem Cell 2013;13:328-40. [Crossref] [PubMed]
- Lalit PA, Salick MR, Nelson DO, et al. Lineage Reprogramming of Fibroblasts into Proliferative Induced Cardiac Progenitor Cells by Defined Factors. Cell Stem Cell 2016;18:354-67. [Crossref] [PubMed]
- Dubois NC, Craft AM, Sharma P, et al. SIRPA is a specific cell-surface marker for isolating cardiomyocytes derived from human pluripotent stem cells. Nat Biotechnol 2011;29:1011-8. [Crossref] [PubMed]
- Choi SH, Jung SY, Suh W, et al. Establishment of isolation and expansion protocols for human cardiac C-kit-positive progenitor cells for stem cell therapy. Transplant Proc 2013;45:420-6. [Crossref] [PubMed]
- Masino AM, Gallardo TD, Wilcox CA, et al. Transcriptional regulation of cardiac progenitor cell populations. Circ Res 2004;95:389-97. [Crossref] [PubMed]
- Moretti A, Caron L, Nakano A, et al. Multipotent embryonic isl1+ progenitor cells lead to cardiac, smooth muscle, and endothelial cell diversification. Cell 2006;127:1151-65. [Crossref] [PubMed]
- Bondue A, Tännler S, Chiapparo G, et al. Defining the earliest step of cardiovascular progenitor specification during embryonic stem cell differentiation. J Cell Biol 2011;192:751-65. [Crossref] [PubMed]
- Ferreira-Martins J, Ogórek B, Cappetta D, et al. Cardiomyogenesis in the developing heart is regulated by c-kit-positive cardiac stem cells. Circ Res 2012;110:701-15. [Crossref] [PubMed]
- Ellison GM, Vicinanza C, Smith AJ, et al. Adult c-kit(pos) cardiac stem cells are necessary and sufficient for functional cardiac regeneration and repair. Cell 2013;154:827-42. [Crossref] [PubMed]
- Oh H, Bradfute SB, Gallardo TD, et al. Cardiac progenitor cells from adult myocardium: homing, differentiation, and fusion after infarction. Proc Natl Acad Sci U S A 2003;100:12313-8. [Crossref] [PubMed]
- Takamiya M, Haider KH, Ashraf M. Identification and characterization of a novel multipotent sub-population of Sca-1+ cardiac progenitor cells for myocardial regeneration. PLoS One 2011;6:e25265. [Crossref] [PubMed]
- Ye J, Boyle A, Shih H, et al. Sca-1+ cardiosphere-derived cells are enriched for Isl1-expressing cardiac precursors and improve cardiac function after myocardial injury. PLoS One 2012;7:e30329. [Crossref] [PubMed]
- Freire AG, Nascimento DS, Forte G, et al. Stable phenotype and function of immortalized Lin-Sca-1+ cardiac progenitor cells in long-term culture: a step closer to standardization. Stem Cells Dev 2014;23:1012-26. [Crossref] [PubMed]
- Wang H, Chen H, Feng B, et al. Isolation and characterization of a Sca-1+/CD31- progenitor cell lineage derived from mouse heart tissue. BMC Biotechnol 2014;14:75. [Crossref] [PubMed]
- Fu JD, Stone NR, Liu L, et al. Direct reprogramming of human fibroblasts toward a cardiomyocyte-like state. Stem Cell Reports 2013;1:235-47. [Crossref] [PubMed]
- Wada R, Muraoka N, Inagawa K, et al. Induction of human cardiomyocyte-like cells from fibroblasts by defined factors. Proc Natl Acad Sci U S A 2013;110:12667-72. [Crossref] [PubMed]
- Nam YJ, Song K, Luo X, et al. Reprogramming of human fibroblasts toward a cardiac fate. Proc Natl Acad Sci U S A 2013;110:5588-93. [Crossref] [PubMed]
- Song K, Nam YJ, Luo X, et al. Heart repair by reprogramming non-myocytes with cardiac transcription factors. Nature 2012;485:599-604. [Crossref] [PubMed]
Cite this article as: Ii M. Novel direct reprogramming technique for the generation of culture-expandable cardiac progenitor cells from fibroblasts. Stem Cell Investig 2017;4:15.


