Unsaturated fatty acids regulate stemness of ovarian cancer cells through NF-κB
Cancer stem cells (CSCs), also known as tumor-initiating cells (TICs), constitute a small population of cells within the tumor that has abilities to self-renew like normal stem cells and initiate tumor development (1). CSCs are believed to be responsible for the development of distant metastasis, chemotherapy resistance, and tumor relapse (2). Understanding the regulatory mechanisms behind unique characteristics and vulnerabilities of CSCs would accelerate the development of new therapeutic strategies to prevent tumor relapse and metastasis. Studies have revealed stemness-associated signaling pathways, such as Wnt, Sonic hedgehog (Shh), and Notch, as potential targets for CSCs (3). Increasing evidence demonstrates that upregulation of proteins involved in lipogenesis is also associated with stem-like properties of breast and colorectal cancer cells (4-6).
Recently, using hyperspectral-stimulated Raman scattering (SRS) microscopy, Li et al. (7) show that ovarian CSCs identified as ALDH+/CD133+ population have higher levels of unsaturated fatty acids (UFAs) when compared with non-CSC population (ALDH−/CD133−). This finding is further validated using ovarian cancer cells grown in spheroid conditions (non-adherent, serum-free), in which sphere-derived ovarian cancer cells have increased levels of UFAs compare with those observed in monolayer. They also show higher mRNA levels of stearoyl-CoA desaturase-1 (SCD1, also known as desaturase Δ9), an enzyme that produces mono-UFAs, in ALDH+/CD133+ cells sorted from two different ovarian cancer cell lines (OVCAR5 and COV362), when compare with ovarian non-CSC (ALDH−/CD133−) population. Downregulation of SCD1 by shRNA reduces mRNA expression of ALDH1A1 in spheroids derived from these ovarian cancer cell lines (7). Pharmacologic inhibition of SCD1 and Δ6 desaturases which produce mono-UFAs and poly-UFAs, respectively, also reduces the mRNA expression of stem cell markers, ALDH1A1, Sox2, Nanog and Oct-4, in spheroids derived from ovarian cancer cell lines and patients-derived tumor cells (7). These data suggest that SCD1 could be a novel biomarker for ovarian CSCs. It would be interesting to determine the SCD1 expression in human ovarian cancer tissues, especially in the CSC population, and if SCD1 overexpression could enhance stemness in non-CSCs. Furthermore, inhibition of desaturases also reduces spheroid formation and proliferation of ovarian cancer cells, which is not rescue by supplementation with a mono-UFA, oleic acid (7). These data may suggest that de novo synthesis of UFAs is required to enhance stemness in ovarian cancer cells; however, it would be important to determine whether supplementation with a pool of different UFAs could rescue the effects of desaturases inhibitors in ovarian cancer cells.
High tumor initiation potential and resistance to chemotherapy are characteristics of CSCs, and hence CSCs are believed to be responsible for metastasis and tumor relapse (2). Indeed, high levels of CSCs in tumors are well correlated with poor prognosis in ovarian cancer patients (8). Interestingly, pretreatment of ovarian cancer cells with desaturases inhibitors significantly reduces tumor initiation potential and tumor growth in a subcutaneous xenograft mouse model (7). RT-PCR-based signal array analyses reveal that inhibition of lipid desaturation reduces the NF-κB activity in ovarian cancer cells-derived spheroids (7). Moreover, overexpression of the active subunit of NF-κB RelA (p65) increases proliferation of ovarian cancer cells and ALDH1A1 mRNA expression in spheroids, while inhibition of NF-κB reduces expression of ALDH1A1 mRNA (7). Intriguingly, inhibition of NF-κB also reduces SCD1 mRNA expression and UFA levels, while overexpression of RelA (p65) increases spheroid-forming potential of ovarian cancer cells and SCD1 mRNA expression through direct binding to two p65 binding regions in the SCD1 promoter (Figure 1) (7). Additionally, inhibition of ALDH1A1 reduces the levels of UFAs, which is nullified by supplementation with retinoic acid (RA) that is produced by ALDH1A1 (7); however, the mechanism by which RA restores UFA levels remains unclear. These data suggest that UFAs enhance the NF-κB activity, which in turn increases the expression of SCD1 desaturase, thus forming a positive feedback loop (Figure 1).
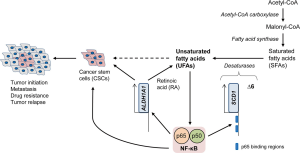
Their findings highlight the importance of lipid metabolism in the tumor initiation, and also suggest that increased lipid unsaturation could be a metabolic marker for ovarian CSCs. Moreover, the UFAs—NF-κB positive feedback loop could be aimed to develop novel therapeutic strategies for targeting ovarian CSCs (Figure 1). However, there are several unanswered questions: (I) how UFAs enhance the transcriptional activity of NF-κB; (II) whether other lipogenic pathways, such as fatty acid biosynthesis and the mevalonate pathway, could also enhance the NF-κB activity; (III) whether NF-κB regulates the expression of other stem cell markers, such as Sox2, Nanog, Oct-4, or CD133; and (IV) if UFAs could increase stemness of ovarian cancer cells through mechanisms other than NF-κB. Additionally, it would be crucial to determine whether the results found in their study can be extrapolated to other cancer types. This includes correlative studies between UFA levels and stem cell marker expression or prognosis in cancer patients, as well as functional studies to test the roles of desaturases in the stemness of cancer cells.
From therapeutic viewpoints, it is important to evaluate the effects of desaturase inhibitors on ovarian cancer progression using several mouse models, as well as perform studies for pharmacodynamics, pharmacokinetics, and adverse effects of these inhibitors. It also remains unclear whether effects of desaturase inhibitors are specific to CSCs or how they impact on non-CSC populations. Given that normal cells usually obtain lipids directly from the bloodstream (9) and cancer cells often have an increased ability of de novo lipid synthesis (10), desaturase inhibitors might specifically affect cancer cells, especially the CSC population, with minimum effects on normal cells.
Acknowledgements
Funding: This work is supported by NIH 1-R01-CA174735-03 (T.I.) grant.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Reya T, Morrison SJ, Clarke MF, et al. Stem cells, cancer, and cancer stem cells. Nature 2001;414:105-11. [Crossref] [PubMed]
- Adhikari AS, Agarwal N, Iwakuma T. Metastatic potential of tumor-initiating cells in solid tumors. Front Biosci (Landmark Ed) 2011;16:1927-38. [Crossref] [PubMed]
- Chen K, Huang YH, Chen JL. Understanding and targeting cancer stem cells: therapeutic implications and challenges. Acta Pharmacol Sin 2013;34:732-40. [Crossref] [PubMed]
- Pandey PR, Okuda H, Watabe M, et al. Resveratrol suppresses growth of cancer stem-like cells by inhibiting fatty acid synthase. Breast Cancer Res Treat 2011;130:387-98. [Crossref] [PubMed]
- Tirinato L, Liberale C, Di Franco S, et al. Lipid droplets: a new player in colorectal cancer stem cells unveiled by spectroscopic imaging. Stem Cells 2015;33:35-44. [Crossref] [PubMed]
- Wang X, Sun Y, Wong J, et al. PPARγ maintains ERBB2-positive breast cancer stem cells. Oncogene 2013;32:5512-21. [Crossref] [PubMed]
- Li J, Condello S, Thomes-Pepin J, et al. Lipid Desaturation Is a Metabolic Marker and Therapeutic Target of Ovarian Cancer Stem Cells. Cell Stem Cell 2017;20:303-14.e5. [Crossref] [PubMed]
- Zhang J, Guo X, Chang DY, et al. CD133 expression associated with poor prognosis in ovarian cancer. Mod Pathol 2012;25:456-64. [Crossref] [PubMed]
- Santos CR, Schulze A. Lipid metabolism in cancer. FEBS J 2012;279:2610-23. [Crossref] [PubMed]
- Beloribi-Djefaflia S, Vasseur S, Guillaumond F. Lipid metabolic reprogramming in cancer cells. Oncogenesis 2016;5:e189. [Crossref] [PubMed]
Cite this article as: Parrales A, Ranjan A, Iwakuma T. Unsaturated fatty acids regulate stemness of ovarian cancer cells through NF-κB. Stem Cell Investig 2017;4:49.


