A case report of congenital glycogen storage liver cirrhosis treated with bone marrow derived stem cells
Introduction
There are several causes of liver cirrhosis spanning autoimmune, viral, congenital, and toxic etiologies. Liver transplantation is the only potentially curative treatment for liver cirrhosis but is riddled with problems including the need for lifelong immune suppression and a long waiting period during which most patients expire especially in the developing countries. The high financial, medical and psychological costs make it out of reach for most patients (1-3). Many alternative approaches are being sought. One of those is cellular therapy; stem cells can be easily collected from the patient bone marrow or expanded ex vivo.
Glycogen storage disease consists of several conditions characterized by a transport defect of glucose-6-phosphate due to a mutation of one of the translocase enzymes. It usually results in neutrophil dysfunction and liver cirrhosis. The bone marrow mononuclear stem cells include several types of hematopoietic, mesenchymal and other precursor cells. There are several proposed mechanisms as to their mode of action. The cells usually home to the injured, inflamed areas as well as back to the bone marrow. Although the ultimate goal is to replace the defective cells by differentiation in a specific microenvironment, there is evidence of immune manipulation and paracrine activities that induce repair and reduce inflammation and fibrosis through several mediators (4-7).
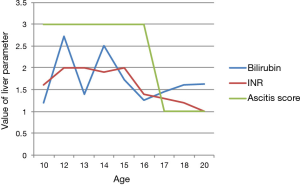
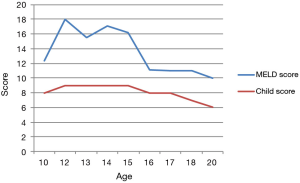
The improvement is usually demonstrated by monitoring the liver function tests, the Child-Pugh and MELD scores, reduction in the ascites buildup, encephalopathy, and the quality of life (8-13).
Case presentation
Our patient is a 21-year-old man who was diagnosed with liver cirrhosis at age 8. At the time, he presented with severe asthenia, very large ascites, failure to thrive and poor appetite. His liver function tests were elevated (Tables 1,2), liver and spleen ultrasonography were compatible with liver cirrhosis. A liver biopsy showed significant triad inflammation, hepatocyte swelling and fibrosis compatible with glycogen storage disease stage 3 on the Ishak scoring system at the time. Since presentation, he was having paracentesis of about 6–8 liters every 5–6 days despite the use of diuretics. The serology for hepatitis A, B and C were negative on repeated occasions.
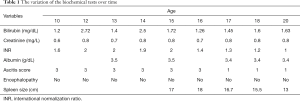
Full table

Full table
At age 16, after 3 days of 300 mcg of G-CSF priming given twice daily, 150 mL of bone marrow was aspirated from the posterior iliac crests using 6 portals with heparin sodium as anticoagulant under general anesthesia. The Bone Marrow was centrifuged and the supernatant fat cells and bone debris were removed.
In the Interventional Radiology Lab, the patient was prepped. Under local anesthesia, the right femoral artery was accessed using a Simon catheter advanced into the descending aorta up to the hepatic artery and the stem cells were infused over 15 minutes. The patient was observed for 24 hours following the procedure.
Following the infusion of the stem cells, the ascites build-up became 2–4 liters every 20–30 days on the average. At age 17, a second infusion of bone marrow derived stem cells was performed again in the Hepatic artery. Following this procedure, the ascites diminished gradually over the following 3 months until no paracentesis was necessary and a very small residue could be detected by ultrasonography with no need for any diuretics or taping.
The patient tolerated the procedures well with no major complications or other effects. No bleeding, hemodynamic instabilities, infectious or other adverse events were reported over the last 4 years of follow up. The blood counts varied slightly after the procedure but no significant cytopenias were noted. The duration of response has been significant and longer than reported in the literature (12-14).
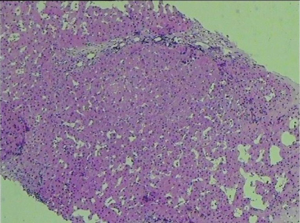
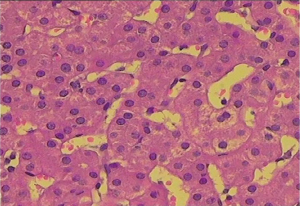
The diuretic requirements of spironolactone and furosemide were variable but mostly high before the procedure and diminished gradually until they were stopped about 3 months following the procedure. A liver biopsy was taken 2 years following the procedure (Figures 1,2) and reviewed by two independent pathologists, it showed again stigmata of hepatocyte swelling, sinus congestion, portal inflammation and fibrosis, however, this was significantly improved compared to the pre-treatment biopsy description. The Ishak liver fibrosis score was downgraded to 1 compared to 3 before the procedures (Figures 3,4). The patient has been on the liver transplant waiting list in the area and is considered stable since.
Discussion
Over the past decade, significant evidence accumulated showing the safety and efficacy of cell therapy in the treatment of liver cirrhosis (7-13). Our case adds one more piece of evidence to show that the bone marrow derived cells given to patients with liver cirrhosis may improve the Child and MELD scores as well as the biochemical tests, and most importantly, the quality of life of the patient correlating with the pathologic improvement. Repeated injections may add up the beneficial effects even though the optimal sequence and schedule is not known (8-12).
This is the first case report showing pathologic evidence along with improvement of the clinical picture of the disease even though one has to keep in mind that many more cases and randomized studies are needed to confirm and verify these results. It is difficult to explain how the autologous cells which carry the same congenital mutations could help a congenital problem except by compensatory and paracrine routes resulting in reduction of the fibrosis. The stem cells have been postulated to regulate the immune cytokines and growth factors to control the liver matrix secreting cells and therefore the process of fibrosis (15-17).
Liao et al. reported giving autologous bone marrow cells to 12 patients with post-hepatitis cirrhosis and portal hypertension through the hepatic artery after in vitro expansion for 7 days. They reported improvement of the liver function tests and stabilization of the liver-related symptoms over the following 6 months (12). Al Tayeb et al. showed efficacy and safety of autologous stem cell transplantation in patients with liver cirrhosis related to hepatitis C infections (13). Yannaki et al. proved the safety and efficacy of mobilized peripheral blood stem cells (PBSCs) in patients with advanced-stage alcoholic liver cirrhosis (14).
In addition to the bone marrow derived stem cells, several groups have studied autologous and allogeneic mesenchymal stem cells (MSCs) and reported positive results (10,18-20). Zhang et al. reported a significant reduction of the volume of ascites and improved liver function tests, albumin, and bilirubin in 30 patients treated with Umbilical cord-derived allogeneic MSCs compared with 15 patients treated with saline as controls (18).
El-Ansary reported on 25 patients with advanced (Child C) liver cirrhosis treated with autologous bone marrow-derived MSCs in patients with chronic hepatitis C infection. Partial objective improvement of liver function tests, prothrombin time, albumin, bilirubin and MELD score were reported (21).
Conclusions
We hope that well designed, large, randomized studies will prove the best types, methods and schedules to get the most out of the stem cells in the future.
In our case, the pathologic proof and improvement of the albumin manufacturing, prothrombin time, liver function tests, splenomegaly, and rate of ascites production and build up are surrogate measures that can be used to assess the effects on patients (19).
It is unclear which pathologies and etiologies may benefit more from this treatment and whether this improvement could serve as a bridge to hold the patients long enough until liver transplants become available or even unnecessary.
Acknowledgements
We acknowledge the contribution of this patient and his family.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Haydon GH, Neuberger J. Liver transplantation of patients in end-stage cirrhosis. Baillieres Best Pract Res Clin. Gastroenterol 2000;14:1049-73. [Crossref] [PubMed]
- Rosen HR. Disease recurrence following liver transplantation. Clin Liver Dis 2000;4:675-89. [Crossref] [PubMed]
- Schuppan D, Afdhal NH. Liver cirrhosis. Lancet 2008;371:838-51. [Crossref] [PubMed]
- Zhang L, Theise N, Chua M, et al. The stem cell niche of human livers: symmetry between development and regeneration. Hepatology 2008;48:1598-607. [Crossref] [PubMed]
- Shi Y, Su J, Roberts AI, et al. How mesenchymal stem cells interact with tissue immune responses. Trends Immunol 2012;33:136-43. [Crossref] [PubMed]
- Keeffe EB. Liver transplantation: current status and novel approaches to liver replacement. Gastroenterology 2001;120:749-62. [Crossref] [PubMed]
- Mimeault M, Hauke R, Batra SK. Stem cells: a revolution in therapeutics-recent advances in stem cell biology and their therapeutic applications in regenerative medicine and cancer therapies. Clin Pharmacol Ther 2007;82:252-64. [Crossref] [PubMed]
- Forbes SJ, Newsome PN. New horizons for stem cell therapy in liver disease. J Hepatol 2012;56:496-9. [Crossref] [PubMed]
- Terai S, Ishikawa T, Omori K, et al. Improved liver function in patients with liver cirrhosis after autologous bone marrow cell infusion therapy. Stem Cells 2006;24:2292-8. [Crossref] [PubMed]
- Mohamadnejad M, Alimoghaddam K, Mohyeddin-Bonab M, et al. Phase 1 trial of autologous bone marrow mesenchymal stem cell transplantation in patients with decompensated liver cirrhosis. Arch Iran Med 2007;10:459-66. [PubMed]
- Sakaida I, Terai S, Nishina H, et al. Development of cell therapy using autologous bone marrow cells for liver cirrhosis. Med Mol Morphol 2005;38:197-202. [Crossref] [PubMed]
- Liao X. Therapeutic effect of autologous bone marrow-derived liver stem cells transplantation in hepatitis B virus-induced liver cirrhosis. Hepatogastroenterology 2013;60:406-9. [PubMed]
- Al Tayeb H, Dorry AE, Amer N, et al. Autologous Stem Cells Transplantation in Egyptian Patients with Liver Cirrhosis on Top of Hepatitis C Virus. Int J Stem Cells 2015;8:209-18. [Crossref] [PubMed]
- Yannaki E, Anagnostopoulos A, Kapetanos D, et al. Lasting amelioration in the clinical course of decompensated alcoholic cirrhosis with boost infusions of mobilized peripheral blood stem cells. Exp Hematol 2006;34:1583-7. [Crossref] [PubMed]
- Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol 2008;8:726-36. [Crossref] [PubMed]
- Akiyama K, Chen C, Wang D, et al. Mesenchymal stem cell induced immunoregulation involves FAS-ligand-/FAS-mediated T cell apoptosis. Cell Stem Cell 2012;10:544-55. [Crossref] [PubMed]
- Luk JM, Wang PP, Lee CK, et al. Hepatic potential of bone marrow stromal cells: development of in vitro co-culture and intra-portal transplantation models. J Immunol Methods 2005;305:39-47. [Crossref] [PubMed]
- Zhang Z, Lin H, Shi M, et al. Human umbilical cord mesenchymal stem cells improve liver function and ascites in decompensated liver cirrhosis patients. J Gastroenterol Hepatol 2012;27:112-20. [Crossref] [PubMed]
- Shi M, Zhang Z, Xu R, et al. Human mesenchymal stem cell transfusion is safe and improves liver function in acute-on-chronic liver failure patients. Stem Cells Transl Med 2012;1:725-31. [Crossref] [PubMed]
- Peng L, Xie DY, Lin BL, et al. Autologous bone marrow mesenchymal stem cell transplantation in liver failure patients caused by hepatitis B: short-term and long-term outcomes. Hepatology 2011;54:820-8. [Crossref] [PubMed]
- El-Ansary M, Abdel-Aziz I, Mogawer S, et al. Phase II trial: undifferentiated versus differentiated autologous mesenchymal stem cells transplantation in Egyptian patients with HCV induced liver cirrhosis. Stem Cell Rev 2012;8:972-81. [Crossref] [PubMed]
Cite this article as: Wehbe TW, Abi Chahine NH, Annous AR, Ferri MA, Boulous RC, El-Mestrah MF. A case report of congenital glycogen storage liver cirrhosis treated with bone marrow derived stem cells. Stem Cell Investig 2017;4:73.