Stem Cell Ophthalmology Treatment Study: bone marrow derived stem cells in the treatment of non-arteritic ischemic optic neuropathy (NAION)
Introduction
Non-arteritic Ischemic Optic Neuropathy (NAION) (1-10) is an ischemic, non-inflammatory process involving the prelaminar, laminar and retrolaminar optic nerve. In the United States, the annual incidence of NAION is 2.3 to 10.2 per 100,000 population. There is no gender predilection, the mean age of occurrence is from 57 to 65 years, and the condition occurs with a significantly higher frequency in the white population than in Hispanic or black individuals.
NAION typically presents with painless visual loss occurring over hours to days. The visual loss is generally static though a progressive form is noted in 22% to 37% of non-arteritic cases. Most cases of NAION are idiopathic, but specific causes such as hypotension and radiation injury have been identified. About 55–80% of the visual field defects demonstrate an altitudinal, generally inferior, loss.
The visual loss from NAION generally remains stable after 2 months following the initial incident. Within 4 to 6 weeks following NAION, the optic disc becomes atrophic, either in a sectoral or diffuse pattern. Involvement of the contralateral eye occurs in 14.7% of patients with a median follow-up of 5 years. Risk factors for second eye involvement were baseline visual acuity of 20/200 or worse and diabetes. Thrombosis has been implicated but not proven as a cause of NAION. If a decrease in perfusion secondary to an obstruction and not a complete occlusion is the instigating factor then his pathologic confirmation will be elusive. Structurally small “crowded” optic discs, the “disc at risk” has been associated with the development of NAION. If a decrease in perfusion leads to axonal swelling, then a compartment syndrome may result leading to further ischemia and the development of NAION.
Histologic human studies support the concept that compromise of the posterior ciliary circulation plays a role in the development of NAION. Though the experimental development of NAION has been seen in monkeys by occluding the posterior ciliary arteries, not all non-human demonstrate permanent or transient damage following this procedure. It has been hypothesized that central retinal artery and pial arterioles anastomoses may prevent ischemia. This supportive mechanism may be absent through normal aging or from systemic disease including hypertension or atherosclerosis.
Optic atrophy represents the morphologic consequence of multiple diseases that damage the ganglion cells and axons. Though inner retinal layer atrophy is seen in eyes with NAION (11) a considerable portion of the retinal nerve fiber layer (RNFL) and the ganglion cell layer (GCC) remain in patients even with no light perception (NLP) vision (12).
Optic disc pallor cannot be directly equated with visual function. Optic disc measurement by ocular coherence tomography (OCT) is an anatomic, not functional test. Visual field testing is a subjective test reflecting visual function.
Treatments for NAION have included anticoagulants, diphenylhydantoin, atropine sulfate, steroids and subtenons injections of vasodilators. None have been proven to be beneficial. The Ischemic Optic Neuropathy Decompression Trial demonstrated that optic nerve sheet decompression was potentially harmful (13).
We have previously reported the treatment with BMSC of 2 patients with optic nerve disease (14,15). The first patient, a 32-year-old female with a 5 year history of visual loss secondary to idiopathic optic atrophy was followed at the Wilmer Eye Institute at the Johns Hopkins Hospital. Pre-treatment best-corrected visual acuity was 20/800 OD and 20/4,000 with severe visual field loss. Four months following surgery, the visual acuity had improved to 20/100 OD and 20/40 OS with improvement in the visual fields OU. At 2 years following initial treatment, and with an intervening second treatment in the Stem Cell Ophthalmology Treatment Study (SCOTS) approximately 13 months after the first, the visual improvements reached 20/40-2 OD and 20/30+2 OS.
The second patient, a 54-year-old female with Neuromyelitis Optica, was followed at the Nationwide Children’s Hospital and The Ohio State University. Pre-treatment, the visual acuity was 20/350 OD and 20/70 OS with severe visual field loss OU. Six months following treatment, the central visual acuity had improved to 20/150 OD and 20/15 OS with improvement in the visual fields OU.
SCOTS is the largest ophthalmology stem cell study registered with the National Institutes of Health: NCT Number 01920867. SCOTS is an open label, non-randomized, efficacy study. There is no placebo or sham arm. All patients meeting eligibility criteria and enrolled in the study receive active treatment. Bone Marrow aspirated from the posterior Iliac Crest is separated to provide Bone Marrow Derived Stem Cells (BMSC) within the stem cell concentrate. Inclusion criteria for SCOTS provide that patients:
- Have objective, documented damage to the retina or optic nerve unlikely to improve OR have objective, documented damage to the retina or optic nerve that is progressive.
- AND have less than or equal to 20/40 best corrected central visual acuity in one or both eyes AND/OR an abnormal visual field in one or both eyes.
- Be at least 3 months post-surgical treatment intended to treat any ophthalmologic disease and be stable.
- If under current medical therapy (pharmacologic treatment) for a retinal or optic nerve disease be considered stable on that treatment and unlikely to have visual function improvement (for example, glaucoma with intraocular pressure stable on topical medications but visual field damage).
- Have the potential for improvement with BMSC treatment and be at minimal risk of any potential harm from the procedure.
- Be 18 years of age or older.
- Be medically stable and able to be medically cleared by their primary care physician or a licensed primary care practitioner for the procedure. Medical clearance means that in the estimation of the primary care practitioner, the patient can reasonably be expected to undergo the procedure without significant medical risk to health.
Exclusion criteria include:
- Patients who are not capable of an adequate ophthalmologic examination or evaluation to document the pathology.
- Patients who are not capable or not willing to undergo follow up eye exams with the principal investigator or their ophthalmologist or optometrist as outlined in the protocol.
- Patients who are not capable of providing informed consent.
- Patients who may be at significant risk to general health or to the eyes and visual function should they undergo the procedure.
There are three arms of SCOTS with the type of treatment chosen based on the degree of visual loss, etiology of visual loss, associated risk factors for the treatment arms and the patient’s medical risk status. Bilateral treatment is provided assuming both eyes meet eligibility requirements. As these are autologous stem cells, no immunosuppression is required. An FDA cleared class 2 medical device is used to separate the bone marrow aspirate into a stem cell concentrate. This concentrate has averaged 1.2 billion Total Nucleated Cells including mesenchymal stem cells in approximately 14–15 cc of concentrate. Retrobulbar injection consists of 3 cc of concentrate; subtenons injection of 1 cc; intravitreal injection of 0.05 cc; subretinal injection of approximately 0.1 cc and intra-optic nerve injection of approximately 0.1 cc. The intravenous injection is provided from the remainder of the concentrate.
Arm 1 consists of stem cell concentrate injected retrobulbar and subtenons followed by intravenous infusion. Patients with ophthalmic conditions which preclude safe or effective utilization of intravitreal injection of concentrate, such as the presence of silicone oil, may be offered Arm 1 if meeting inclusion criteria. Arm 2 consists of the administration of retrobulbar, subtenon and intravitreal concentrate followed by intravenous infusion. Patients meeting inclusion criteria with visual acuity between 20/40 and 20/200 in one or both eyes and/or visual field loss may be offered Arm 2. Arm 3 is reserved for retinal and optic nerve patients with severe visual loss typically meaning visual acuity of 20/200 or worse in at least one eye. Arm 3 consists of the eye with better visual acuity receiving the same treatment as Arm 1 or more typically, Arm 2, and the eye with more extensive visual acuity loss receiving a core pars plana vitrectomy (PPV) with injection of subretinal or intra-optic nerve concentrate followed by the infusion of intravenous stem cells. Monocular patients are not eligible for Arm 3. Follow up is required at 1, 3, 6 and 12 months post treatment with reporting of the eye exam results to the Principal Investigator and Study Director.
Unlike pharmaceutical studies in which a specific condition is treated, SCOTS focuses on the endpoint of visual loss, not the instigating cause. This allows the treatment of various retinal and optic nerve diseases, many of which are not presently the subject of clinical treatment studies, and include visual loss resulting from more than one disease.
The SCOTS procedure is patient funded and typically performed under general anesthesia. Treatment is provided in a fully licensed ambulatory surgical center in Coconut Creek, Florida.
Clinical histories
Patient 1
In 1989, this 46-year-old male experienced an acute visual loss in his right eye (OD) to counting fingers centrally with a right Marcus-Gunn afferent pupil defect. There was an altitudinal visual field defect and optic disc swelling consistent with NAION. Hematologic testing did not demonstrate vasculitis or coagulopathy risk factors. The patient’s medical history was negative for diabetes, hypertension or hyperlipidemia. In 1999, at 56 years of age, he experienced visual loss of the left eye (OS) to 20/50 with arcuate and altitudinal visual field defects and disc edema OS. The visual field defects resulted in the loss of his driver’s license and the loss of his manufacturing job.
In 2014, the visual acuities were stable at Count Fingers (CF) at 2 feet OD and 20/40 OS. The visual fields were unchanged from 1999. The patient was assigned to SCOTS Arm 3 OD and Arm 2 OS. Preoperative Snellen vision was 20/400 OD, pinhole (PH) no improvement (NI), and 20/50+ OS, PH NI. BMSC were administered directly into the optic nerve by vitrectomy and subtenons OD and retrobulbar, subtenons and intravitreal OS on December 12, 2014 without complication. Examination at 1, 3, and 6 months demonstrated improvement in visual acuity, peaking at 6 months with best corrected Snellen acuities of 20/150 OD and 20/30+ OS. Improvements in the Humphrey and Goldmann visual field were also observed. The macular volume measurements by OCT had peaked in thickness at 9 months post-SCOTS in each eye.
Patient 2
A 54-year-old male with a history of buried optic disc drusen acutely lost vision OD in 1980 with central scotomas and arcuate defects. An edematous optic nerve head typical of NAION was noted. Hematologic testing was unremarkable.
Twenty-seven years later, at 81 years of age, he acutely lost vision OS, similar to OD. The visual acuity was 20/400 OD and CF at 6 feet OS. In 2012, at age 86, the patient underwent cataract surgery OD. One week later he suffered a second attack of NAION OD associated with an intraocular pressure (IOP) spike to 43. Despite normalization of the IOP, over the next 10 days the vision OD declined rapidly to hand motions (HM). A radial optic neurotomy OD was performed and the visual acuity improved to 20/100 OD. Visual fields demonstrated central scotomas and both superior and inferior arcuate defects OU. There was no spontaneous improvement over the following 2 years. He chose to participate in the SCOTS study on 10/7/14. Preoperative Snellen vision was 20/200 OD, PH NI, and CF 3 feet OS, PH NI. The patient was assigned to Arm 2 OD and Arm 3 OS and the procedures were performed without complication. The Humphrey visual field demonstrated improvement at 3 months OD and OS was unchanged. The maculae demonstrated thickening by OCT at 3 months. At 9 months, the best corrected Snellen visual acuity OD was 20/100-1 and OS remained CF 3 feet.
Patient 3
A 56-year-old male experienced an acute decrease in visual acuity OD in 2004. The visual acuity OD was 20/40, an inferonasal visual field defect was noted, and dilated fundus examination demonstrated a swollen optic disc, typical of NAION. Hematologic evaluation revealed a heterozygous positive Leidin Factor V and a homozygous positive MTHFR mutation. He was treated with aspirin and folic acid. In 2013, he experienced acute visual loss OS to counting fingers. The patient was assigned to SCOTS Arm 2 OD and Arm 3 OS. Preoperative Snellen vision was 20/30-2 OD, PH NI, and CF 6 feet OS, PH NI. On 10/21/14, he underwent the procedure. One year postoperatively, the Snellen visual acuity OD was 20/30-1. Vision was CF 3 feet OS. There was no change in the Humphrey visual field mean deviation scores on static perimetry OU. OCT at 12 months post-SCOTS showed improved macular volume thicknesses OU slowly improving from month 4 to 12 OD, and immediately improved at 2 weeks OS, the improvement persisting at 12 months.
Patient 4
In 1997, 3 days after cataract surgery, a 41-year-old male suddenly lost the vision OS. His medical history was significant for a 10-year history of diabetes complicated by proliferative diabetic retinopathy OU, treated with pan-retinal photocoagulation, and cataracts. On examination, disc edema, consistent with NAION was noted. A subsequent increase in IOP to 44 mmHg resulted in NLP vision OS. In 2004, he was diagnosed with poorly controlled hypertension and OD developed diabetic macular edema treated by focal grid laser. Cataract surgery in 2008 was complicated postoperatively by sudden visual loss OD and disc edema consistent with NAION. From 2008 to the present, he developed glaucoma OD that failed medical therapy and required a trabeculectomy using mitomycin C on 5/3/12. Postoperative hypotony was treated with topical Lotemax daily. The NLP OS remained on Combigan bid and had pressures from 28 to 56 mmHg with no pain. Progressive visual field testing in 2012, 2013, and 2014 showed loss of the tiny central island of remaining vision OD (mean deviation −25.2 to −26.6 to −31.86, from 15 spots with vision down to 5 spots). Serial OCT of the circular average retinal nerve fiber layer (RNFL) thickness declined from 51 microns in 2013 to 33 microns in 2014 on 8/7/14. The visual acuity OD was 20/70, and the optic nerves appeared cupped OU. Tensions by applanation tonometry were 02 OD and 38 OS. Visual field by Goldmann was 8 degrees nasal with the V4 target and 11 degrees temporal OD. At age 57, he underwent SCOTS Arm 2 OD only on 10/21/14. Preoperative Snellen vision was 20/60-2 OD, PH 20/50-2, and NLP OS. His left eye (NLP since 1997) was untreated. At the 7-month follow-up examination, the patient’s Snellen visual acuity had improved to 20/50+2. Goldmann visual field now saw the V4 target 12 degrees nasally and 25 degrees temporally. His eye pressures were stable at 03 OD and 28 OS on the Lotemax OD daily and Combigan OS bid. The OCT of the right eye pre-operatively had an average RNFL of 33, and at 7 months post-SCOTS it was 43.01 microns. Macular thickness volume pre-operatively had been 5.103 microns and at 7 months post-operatively it measured 5.140.
Patient 5
A 53-year-old male with impaired renal function experienced an acute loss of vision OD in 2000 to 20/400 with altitudinal visual defects and disc edema, typical of NAION. Nine years later he developed gout and started allopurinol, developed acute renal failure and required renal transplantation on 8/21/09. Late in 2009 the patient underwent cataract surgery OD and his visual acuity improved to 20/200. In 2010, he suddenly lost vision OS to counting fingers secondary to NAION. Over the next 5 years, he remained legally blind OU with acuities of 20/200 OD and 20/400 OS. On 12/2/14, he underwent surgery under the SCOTS protocol: OD Arm 2 and OS Arm 3. Preoperative Snellen vision was 20/100-1 OD, PH NI, and 20/200 OS, PH NI. Six months postoperatively his visual acuity was 20/200 OU. Eight months postoperatively, the visual acuity was 20/80 OD and 20/200 OS. OD identified 15 of 19 Ishihara color plates correctly. Humphrey visual fields were more reliable and his mean deviations were −19.30 OD with a superior altitudinal and inferonasal defect splitting fixation and −23.03 OS with both superior altitudinal and inferonasal defects. His OCT macular volumes were 5.762 OD and 7.075 OS. He was able to obtain a drivers license following treatment in SCOTS.
Patient 6
A 75-year-old female had a 30-year history of type 2 diabetes and ulcerative colitis, and a 10 year history of hypothyroidism. In 2005, she developed congestive heart failure and acutely lost vision OD. The patient underwent cataract surgery later that year OU and postoperatively her visual acuities were 20/40 OD and 20/60 OS. There was a right afferent pupil defect and no color vision consistent with NAION OD, and the left with macular pigment disruption.
In 2008, she acutely lost vision OS and was diagnosed with NAION. Hematologic evaluation was negative for vasculitis or coagulopathy. There was a left Marcus- Gunn afferent pupil defect. The visual acuity was 20/150 OD and HM OS, and her left disc was edematous. Retinal examination demonstrated age-related macular degeneration, OS more prominent than OD, and microaneurysms with leakage from background diabetic retinopathy OU. Later in 2008, she noted a new blind spot OS. Subretinal fluid was noted at the left macula, and she was treated with three intravitreal injections of Avastin. In 2012, she again lost vision and now was unable to read. She had a new right homonymous hemianopsia with dyslexia. A brain CT scan showed no hemorrhage but multifocal areas of ischemia in both hemispheres and a left temporal focal lesion. On 8/12/14, the visual acuity was 20/100 OD and CF at 1 foot OS with a left afferent pupillary defect. She was unable to see the Ishihara color plates with either eye. She was also unable to perform the Humphrey visual field with either eye, but the Goldmann kinetic visual field showed a right homonymous hemianopia and small left homonymous islands OU. At 84 years of age, she enrolled in SCOTS and on 12/2/14 underwent Arm 2 stem cell therapy OU. Preoperative Snellen vision was CF 4 feet OD, PH NI, and CF 3 feet OS, PH NI. At 1 month postoperatively, her right eye saw 8/10 color plates. At three months postoperatively, her right homonymous was now predominantly a superior quadrantanopsia, she was able to see and read J (Jaeger) 5, the first near reading in almost 5 years. At the 6 month postoperative visit Snellen acuities were 20/150 OD and 20/400 OS. There was improvement in the visual fields in both homonymous hemifields. At 3 months the macular OCT had an average volume of 6.028 OD and 5.896 OS. This was an improvement over the pre-operative macular OCT OD but a slight loss in the left eye’s macular thickness.
Patient 7
A 50-year-old male with type 2 diabetes and hypertension, acutely lost vision OD in 1997 from NAION. No infectious or inflammatory trigger was found. In 2007 he was diagnosed with a myocardial infarct and required four stents. He was treated with amiodarone for an ongoing arrhythmia. Months later, the patient lost color vision in both eyes and acutely lost vision OS associated with disc swelling. He was diagnosed with a combination of both papilledema and left NAION. Amiodarone was discontinued, an MRI did not show an acute infarct, and a spinal tap was performed with an opening pressure of 27 cm. The vision remained at 20/250 OD and CF at 2 feet OS for the next 8 years. On 5/12/15, he underwent surgery according to the SCOTS protocol: Arm 2 OD, Arm 3 OS. Preoperative Snellen vision was 20/400 OD, PH NI, and CF 4 feet OS, PH NI. 1 week post-operatively the visual acuity was 20/250 OD and HM OS. At 1 month post-operatively the visual acuity was 20/200 OD and CF at 6 inches OS. At the 4-month postoperative examination the Snellen visual acuity was unchanged at 20/200 OD, but the patient was able to perform the Humphrey visual field for the first time OU. The mean deviation OD was −29.63 and −29.58 OS. On OCT, his right macular volume was 5.792 and the left was similar to the 1 month thickness of 5.646. At 13 months the best corrected Snellen acuity OS was CF 3 feet.
Patient 8
A 62-year-old male with a history of hypertension developed NAION of the left eye in 2004 followed by the right eye in 2013. In 2014, corrected visual acuity was noted to be CF at 4 feet in the right eye and 20/80 in the left eye. Ishihara plates showed 0/14 in the right eye and 7/14 in the left. He was evaluated for participation in SCOTS and on pre-operative exam was found to be CF at 6 feet (20/1,333) right eye and 20/100-1 left eye. The right eye received Arm 3. The left eye received Arm 2. Post-operative visit at 5 weeks showed corrected vision right eye to be 20/480-2 and 20/80-1. Post-operative visit at 10 weeks showed corrected visual acuity to be 20/480-4 in the right eye, a 1 line improvement.
Patient 9
A 73-year-old male with NAION was diagnosed in 2004 primarily with peripheral vision impairment and reduced central vision. There had been consideration of amiodarone toxicity as it had been prescribed in 2003 following an initial aortic dissection. Toxicity typically includes the presence of optic disc edema and an improvement in acuity after discontinuation of the drug. In this patient no optic disc edema was noted nor was there subsequent improvement suggesting the diagnosis was NAION. In 2016 the patient elected to participate in SCOTS. Preoperatively the vision was right eye HM and the left 20/50+1. The patient underwent Arm 2 in each eye. On the 3-month visit the cycloplegic refraction on the right eye showed 20/60+2 and the left eye was unchanged at 20/50+2. The change on the right represented an approximately 14 lines improvement.
Patient 10
A 63-year-old male was initially diagnosed with NAION in 2009. He had a history of poorly controlled hypertension and low hemoglobin from possible gastrointestinal bleeding prior to the onset of his visual loss. Vision at that time was recorded as right eye 20/200 and left eye 20/20 without correction on near card. CT scan at that time showed small vessel ischemic changes. In 2013 his vision had deteriorated to CF OU. In 2015 he elected to participate in SCOTS. Preoperatively the acuity was CF at 5 feet in the right eye (20/1,600) and 20/400 in the left eye with NI with PH.
Nearly complete visual field loss was present in both eyes. He underwent the SCOTS procedure: Arm 3 for the right eye and Arm 2 for the left eye. At 8-month post-operative visit the patient’s uncorrected acuity was 20/300 in each eye, an approximately 7 lines improvement in the right eye and a 1 line improvement in the left eye.
Discussion
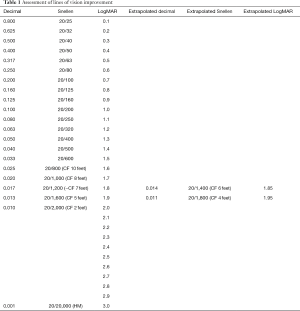
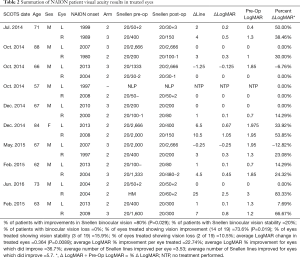
The hope of regenerative medicine is to understand and control human cell function leading to advances in the treatment of presently untreatable medical conditions. In this report the average patient age was 69.8 years. The average period of time between onset of NAION in an eye and treatment in SCOTS was 9.8 years and ranged from 1 to 35 years. Snellen visual acuity lines of vision change were derived from LogMAR conversion. CF or HM acuity was converted to Snellen and LogMAR with extrapolation if needed (Table 1) (16). Following treatment of NAION in SCOTS, 73.6% of eyes treated gained Snellen vision (P=0.019). There was an average of 3.53 Snellen lines of vision gained for each eye at peak and 5.7 lines in those eyes that did improve. An average of 89.5% of individual eyes treated experienced gains or maintained stability in Snellen visual acuity. Two eyes (10.5%) with CF vision lost vision: 1.25 lines for a CF 6 feet eye and 0.25 lines in a CF 3 feet eye. However, their fellow eyes either maintained (20/30-1) or gained 1 line (20/400 to 20/200) respectively, leaving the patients with the same or increased ability to function visually. When considering bilateral vision (binocular Snellen) 80% of patients experienced an increase in vision (P=0.029); 20% maintained binocular stability. No patient showed a loss of binocular Snellen visual acuity in comparison to pre-operative vision. Average LogMAR improvement was 22.74% per eye and for eyes that improved, there was an average of 36.7% improvement on LogMAR acuity. LogMAR improvement ranged up to 83.3% for a HM eye. The average LogMAR change in treated eyes was an increase of 0.364 which was highly significant (P=0.0089). Eyes treated in Arm 3 gained an average of 0.196 LogMAR while those treated in Arm 2 gained an average of 0.443 LogMAR. Comparing eyes in patients who had one eye treated in Arm 2 and one in Arm 3, 42.9% showed greater improvement in the Arm 3 eye while 57% showed greater improvement in the Arm 2 eye. However the eyes treated in Arm 3 had poorer visual acuity pre-operatively and this may have impaired the potential of the eyes to improve (Table 2).
Full table
Full table
Subjective and quality of life changes ranged from stunning joy (2 of the 10 patients, both able to get a new driver’s license), to gratefulness regarding improved brightness, clarity, return of color vision, expanded visual field and ability to ambulate. BMSC have been investigated in the treatment of various conditions including orthopedic, cardiovascular, neurologic and ocular diseases. BMSCs are capable of differentiating into neuron-like cells (17-20). They have been demonstrated to provide neurotrophic factors that promote the regeneration of axons and protect retinal ganglion cells (RGC) and to integrate into existing neural networks thus re-establishing neural connections. Treatment with autologous BMSCs avoids the need for immunosuppression, does not result interatomic formation, and has no ethical or moral objections (21). In a rat model of glaucoma, the BMSCs, following transplantation into the vitreous cavity, did not engraft, but there was a 10–20% increase in the number of surviving RGC (22,23). In a transection/crush model of the optic nerve, BMSC doubled the number of regenerating axons 100–1,200 µm distal to the lesion site compared to a control group. In addition, RGC survival increased by 15–28%, 8–28 days after the injury (24-26). The authors concluded that the neuro protective effects were achieved through retinal glia activation and glia-mediated neuro protection or through signaling between the stem cells and the damaged RGCs. BMSCs have been detected months after implantation into the vitreous cavity of the eye although no retinal engraftment was detected (27). Johnson et al. determined that the retinal glia is the barrier to retinal engraftment indicating that the direct placement of BMSCs into the retina or optic nerve may be of benefit (28,29). In a chemically induced rotenone murine model of LHON, Mansergh et al. suggested that the use of stem cells would be capable of protecting visual function. They noted that cultured retinal progenitor cells can integrate close to the ganglion cell layer and maintain retinal function as ascertained by manganese-enhanced magnetic resonance imaging (MRI) (30). The implication of visual improvement following NAION is that some cells are capable of “healing” following the initial incident. The possibility exists that at the margin of the dead cells are cells that are not functional but not dead and are capable of being “reactivated”.
There have been a number of mechanisms identified for the effects of BMSC including MSC-derived exosomes providing microRNA (31,32), presence of growth factors including brain-derived neurotrophic growth factor (BDNF) (33) nerve growth factor (NGF), glial cell line-derived neurotrophic factor (GDNF) (34), paracrine effects (35,36) as well as transdifferentiation of the stem cells. Vaquero and Zurita [2009] reviewed neuronal transdifferentiation of BMSC and considered the presence of neurotrophic factors in the local environment to be important for in vivo transdifferentiation. They cited previous work in which they identified ascendant and descendent axons in regenerated nervous tissue following intralesional spinal cord administration of BMSC in a murine model (37). Intravitreal BMSC as provided in SCOTS were identified by Kim et al. [2016] as CD34+ and could be seen transdifferentiating into NeuN-positive neuronal cells (38). As noted by Mullen et al. [1992], NeuN is a neuron specific nuclear protein considered a marker for neurons of the central and peripheral nervous system (39). Lin et al. [2013] reported that BMSC promoted neurite outgrowth through neurotrophic factors in vitro including BDNF, NGF and GDNF (40). Our opinion is that, depending on the disease mechanisms, one or more of these methods may predominate and provide a beneficial outcome in various retinal and optic nerve disease.
This report demonstrates that eyes with NAION of many years duration may be capable of recovery of vision through the delivery of autologous BMSC by methods developed in the Stem Cell Ophthalmology Treatment Study (SCOTS). Statistically significant improvements in the visual acuity of individual eyes and of binocular vision in this condition have been shown.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation. All appropriate patient consent for publication of this work has been obtained.
References
- Walsh and Hoyt’s Clinical Neuro-Ophthalmology: The Essentials, Second Edition. Philadelphia: Lippincott Williams & Wilkins, 2008.
- Bernstein SL, Johnson MA, Miller NR. Nonarteritic anterior optic neuropathy (NAION) and its experimental models. Prog Retin Eye Res 2011;30:167-87. [Crossref] [PubMed]
- Atkins EJ. Nonarteritic anterior ischemic optic neuropathy. Curr Treat Options Neurol 2011;13:92-100. [Crossref] [PubMed]
- Mercado JL, Purvin VA, Kawasaki A, et al. Bilateral sequential non-arteritic anterior ischemic optic neuropathy: a comparison of visual outcomes in fellows eyes using quantitative analysis of gold man visual fields. Arch Ophthalmol 2012;130:863-7. [Crossref] [PubMed]
- Chen T, Song D, Shan G, et al. The association between diabetes mellitus and non-arteritic anterior ischemic optic neuropathy: a systematic review and meta-analysis. PLoS One 2013;8:e76653. [Crossref] [PubMed]
- Kernstock C, Beisse F, Wiethoff S, et al. Assessment of functional and morphometric endpoints in patients with non-arteritic anterior ischemic optic neuropathy (NAION). Graefes Arch Clin Exp Ophthalmol 2014;252:515-21. [Crossref] [PubMed]
- Dickersin K, Manheimer E, Li T. Surgery for nonarteritic anterior ischemic opticneuropathy. Cochrane Database Syst Rev 2012;1:CD001538. [PubMed]
- Katz DM, Trobe JD. Is there treatment for nonarteritic anterior ischemic optic neuropathy. Curr Opin Ophthalmol 2015;26:458-63. [Crossref] [PubMed]
- Parsa CF, Hoyt WF. Nonarteritic anterior ischemic optic neuropathy (NAION): a misnomer. Rearranging pieces of a puzzle to reveal a nonischemic papillopathy caused by vitreous separation. Ophthalmology 2015;122:439-42. [Crossref] [PubMed]
- Miller NR, Arnold AC. Current concepts in the diagnosis, pathogenesis and management of nonarteritic anterior ischemic optic neuropathy. Eye (Lond) 2015;29:65-79. [Crossref] [PubMed]
- Rebolleda G, Sanchez-Sanchez C, Gonzalez-Lopez JJ, et al. Papillomacular bundle and inner retinal thicknesses correlate with visual acuity in nonarteritic anterior ischemic optic neuropathy. Invest Ophthal Vis Sci 2015;56:682-92. [Crossref] [PubMed]
- Miki A, Endo T, Morimoto T, et al. Retinal nerve fiber layer and ganglion cell complex thicknesses measured with spectral-domain optical coherence tomography in eyes with no light perception due to nonglaucomatous optic neuropathy. Jpn J Ophthalmol 2015;59:230-5. [Crossref] [PubMed]
- Dickersin K, Li T. Surgery for nonarteritic anterior ischemic optic neuropathy. Cochrane Database Syst Rev 2015;12:CD001538. [PubMed]
- Weiss JN, Levy S, Malkin A. Stem cell ophthalmology treatment trial (SCOTS) for retina and optic nerve disease: a preliminary report. Neural Regen Res 2015;10:982-8. [Crossref] [PubMed]
- Weiss JN, Levy S, Benes SC. Stem cell ophthalmology treatment trial (SCOTS) for retina and optic nerve disease: Case report of improvement in relapsing auto-immune optic neuropathy. Neural Regen Res 2015;10:1507-15. [Crossref] [PubMed]
- Holladay JT. Proper Method for Calculating Average Visual Acuity. J Refract Surg 1997;13:388-91. [PubMed]
- Dezawa M, Takahashi I, Esaki M, et al. Sciatic nerve regeneration in rats induced by transplantation of in vitro differentiate bone marrow stromal cells. Eur J Neurosci 2001;14:1771-6. [Crossref] [PubMed]
- Montzka K, Lassonczyk L, Tschoke B, et al. Neural differentiation potential of human bone marrow-derived mesenchymal stromal cells: misleading marker gene expression. BMC Neurosci 2009;10:16. [Crossref] [PubMed]
- Wilkins A, Kemp K, Ginty M, et al. Human bone marrow-derived mesenchymal stem cells secrete brain-derived neu-rotrophic factor which promotes neuronal survival in vitro. Stem Cell Res 2009;3:63-70. [Crossref] [PubMed]
- Zarzeczny A, Caulfield T. Emerging ethical, legal and social issues associated with stem cell research and the current role of the moral status of the embryo. Stem Cell Rev 2009;5:96-101. [Crossref] [PubMed]
- Yu S, Tanabe T, Dezawa M, et al. Effects of bone marrow stromal cell injection in an experimental glaucoma model. Biochem Biophys Res Commun 2006;344:1071-9. [Crossref] [PubMed]
- Johnson TV, Bull ND, Hunt DP, et al. Neuroprotective effects of intravitreal mesenchymal stem cell transplantation in experimental glaucoma. Invest Ophthalmol Vis Sci 2010;51:2051-9. [Crossref] [PubMed]
- Levkovitch-Verbin H, Sadan O, Vander S, et al. Intravitreal injections of neurotrophic factors secreting mesenchymal stem cells are neuroprotective in rat eyes following optic nerve transection. Invest Ophthalmol Vis Sci 2010;51:6394-400. [Crossref] [PubMed]
- Mead B, Logan A, Berry M, et al. Paracrine-mediated neuroprotection and neuritogenesis of axotomised retinal ganglion cells by human dental pulp stem cells: comparison with human bone marrow and adipose-derived mesenchymal stem cells. PLoS One 2014;9:e109305. [Crossref] [PubMed]
- Mesentier-Louro LA, Zaverucha-do-Valle C, da Silva-Junior AJ, et al. Distribution of mesenchymal stem cells and effects on neuronal survival and axon regeneration after optic nerve crush and cell therapy. PLoS One 2014;9:e110722. [Crossref] [PubMed]
- Mead B, Logan A, Berry M, et al. Intravitreally transplanted dental pulp stem cells promote neuroprotection and axon regeneration of retinal ganglion cells after optic nerve injury. Invest Ophthalmol Vis Sci 2013;54:7544-56. [Crossref] [PubMed]
- Johnson TV, Bull ND, Martin KR. Identification of barriers to retinal engraftment of transplanted stem cells. Invest Ophthalmol Vis Sci 2010;51:960-70. [Crossref] [PubMed]
- Mead B, Scheven BA. Mesenchymal stem cell therapy for retinal ganglion cell neuroprotection and axon regeneration. Neural Regen Res 2015;10:371-3. [Crossref] [PubMed]
- Mansergh FC, Chadderton N, Kenna PF, et al. Cell therapy using retinal progenitor cells shows therapeutic effect in a chemically-induced rotenone mouse model of Leber hereditary optic neuropathy. Eur J Hum Genet 2014;22:1314-20. [Crossref] [PubMed]
- Kordelas L, Rebmann V, Ludwig AK, et al. MSC-derived exosomes: a novel tool to treat therapy refractory graft-versus-host disease. Leukemia 2014;28:970-3. [PubMed]
- Fernández-Messina L, Gutiérrez-Vázquez C, Rivas-García E, et al. Immunomodulatory role of microRNAs transferred by extracellular vesicles. Biol Cell 2015;107:61-77. [Crossref] [PubMed]
- Wilkins A, Kemp K, Ginty M, et al. Human bone marrow-derived mesenchymal stem cells secrete brain-derived neurotrophic factor which promotes neuronal survival in vitro. Stem Cell Res 2009;3:63-70. [Crossref] [PubMed]
- Chen Q, Long Y, Yuan X, et al. Protective effects of bone marrow stromal cell transplantation in injuredrodent brain: Synthesis of neurotrophic factors. J Neurosci Res 2005;80:611-9. [Crossref] [PubMed]
- Mead B, Berry M, Logan A, et al. Stemcell treatment of degenerative eye disease. Stem Cell Res 2015;14:243-57. [Crossref] [PubMed]
- Huang W, Lv B, Zeng H, et al. Paracrine Factors Secreted by MSCs Promote Astrocyte Survival Associated with GFAP Downregulation After Ischemic Stroke Via p38MAPK and JNK. J Cell Physiol 2015;230:2461-75. [Crossref] [PubMed]
- Nagra PK, Foroozan R, Savino PJ, et al. Amiodarone induced optic neuropathy. Br J Ophthalmol 2003;87:420-22. [Crossref] [PubMed]
- Vaquero J, Zurita M. Bone marrow stromal cells for spinal cord repair: A challenge for contemporary neurobiology. Histol Histopathol 2009;24:107-16. [PubMed]
- Kim JY, You YS, Kim SH, et al. Epiretinal membrane formation after intravitreal autologous stem cell implantation in a retinitis pigmentosa patient. Retin Cases Brief Rep 2017;11:227-31. [Crossref] [PubMed]
- Mullen RJ, Buck CR, Smith AM. NeuN, a neuronal specific nuclear protein in verte-brates. Development 1992;116:201-11. [PubMed]
- Lin W, Li M, Li Y, et al. Bone marrow stromal cells promote neurite outgrowth of spinal motor neurons by means of neurotrophic factors in vitro. Neurol Sci 2014;35:449-57. [Crossref]
Cite this article as: Weiss JN, Levy S, Benes SC. Stem Cell Ophthalmology Treatment Study: bone marrow derived stem cells in the treatment of non-arteritic ischemic optic neuropathy (NAION). Stem Cell Investig 2017;4:94.


