Integrin α7: a major driver and therapeutic target for glioblastoma malignancy
Glioblastomas, or glioblastoma multiforme tumors (GBM), are aggressive high-grade malignant gliomas. They are the most frequent brain tumors in adults, corresponding to 12% to 15% of all intracranial tumors and 50% to 60% of astrocytic tumors (1). GBM are heterogeneous tumors caused by mutations in epidermal growth factor receptor (EGFR), isocitrate dehydrogenase (IDH) and platelet derived growth factor receptor alpha (PDGFRA) genes (1). Recent reports support the notion that GBM growth is mediated by stem cells [GBM stem cells (GSCs)] (2-4) that express neural stem cell markers (2) and can differentiate into pericytes and endothelial cells to support tumor vascularization (3,4). Different markers have been described for specific GSC sub-populations (2-4), but no common stem cell marker has been defined yet. Identification of GSC stem cell markers is critical for the development of new therapeutic targets for GBM.
In order to define specific GSC markers, Haas et al. [2017] generated a hybridoma library of antibodies that strongly react to primary GSCs (5). The authors identified one clone, 1.4A12, which robustly binds to different primary GBM lines and identified its ligand to be integrin α7. The monoclonal antibody 1.4A12 binds and blocks integrin α7 signaling in the presence of laminin. Integrins are heterodimeric cell surface proteins composed of α and β subunits that act as mechanosensors and mechanotransducers of the extracellular environment (6). Integrin α7β1 heterodimer is a major laminin receptor in skeletal and cardiac muscle (7) and mutations affecting ITGA7 result in congenital myopathy (8). In cancer, several studies underpin the role of integrin α7 as a tumor suppressor in GBM and other types of tumors (9-11). Haas et al. [2017] demonstrate that integrin α7 is enriched in undifferentiated GSCs in the perivascular regions and downregulated in cells expressing typical neuronal differentiation markers. In vivo orthotopic implantations of xenograft-derived cells revealed that high integrin α7-cells are more proliferative and invasive, suggesting that these undifferentiated GSCs constitute a group of highly aggressive cancer cells. This hypothesis is supported by Jiang et al. [2017] studies, where the authors demonstrated that glioma malignancy is dependent on the cell of origin (12). Jiang et al. [2017] showed that undifferentiated cell-derived tumors become more invasive and aggressive when compared to more differentiated, nestin- and glial fibrillary acidic protein (GFAP)-positive cell-derived tumors. To describe the mechanism underlying integrin α7 function in GSCs, Haas et al. [2017] provided evidence that GSC proliferation is mediated by downstream integrin mediator Akt, whereas invasiveness is regulated by FAK and Src (Figure 1). Akt is a well-known regulator of cell proliferation and is involved in cell-cycle progression by inducing G1/S (13) and G2/M cell cycle transitions (14) and its proposed role on GSC proliferation is consistent with a recent report demonstrating that Akt activation induces glioma cell proliferation and invasion (15). FAK and Src are downstream components of the protein complex that couples the integrin β-cytoplasmic tail to the actin cytoskeleton that act as primary regulators of cell motility (16). Together, the knowledge of the signaling properties of integrin α7 effectors FAK, Src, and Akt supports the involvement of integrin α7 in the basic cellular processes driving GCS mediated GBM progression.
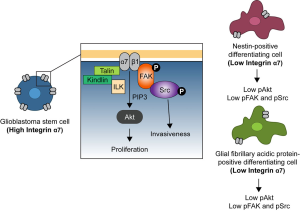
To test the potential for integrin α7 as a therapeutic target for GBM, Haas et al. [2017] performed intracranial brain xenografts of intermediate or high integrin α7 expressing GSCs and then treated the mice with the 1.4A12 antibody. Antibody treatment impaired tumor growth, invasiveness and reduced the number of proliferating cells with active FAK and Akt, extending the survival of the mice. These results suggest that the high malignant profile of GSCs is regulated by integrin α7 and therapeutics targeting integrin α7 might generate successful treatments for GBM patients. Remarkably, the treatment success was based on a local therapy in contrast to most cancer therapies, which are based on systemic approaches that target several tissues and display many side effects.
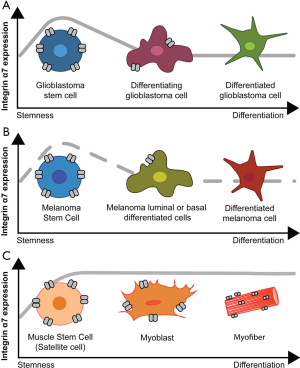
Haas et al. [2017] provide strong in vitro and in vivo evidence that integrin α7 acts as a tumor adjuvant in GBM malignancy by promoting GSC proliferation and migration. A similar role for integrin α7 has been described in oesophageal squamous carcinoma stem cells as a high incidence of integrin α7-positive cells in oesophageal squamous cell carcinoma tissues result in increased lymph node metastasis and worse patient prognosis (17). Interestingly, the role of integrin α7 in proliferation and migration has been described in other cell types including muscle cells. Integrin α7 is highly enriched in muscle stem cells, commonly known as satellite cells, which drive muscle regeneration (7,18). Integrin α7 is defined as a universal marker for satellite cells as reviewed in (19) and its expression is critical for the stem-like cell behavior and binding to laminin in the basement membrane as reviewed in (18). Integrin α7 is highly expressed throughout myoblast differentiation into myofibers and plays a key role in myofiber survival (7,20). The role of integrin α7 in satellite cell proliferation and migration appears to be shared between satellite cells and GSC. Strikingly, integrin α7 function in stem cells contrasts with its role in differentiated muscle and GBM cells as integrin α7 is critical for myofiber survival, but downregulated in GBM cells (Figure 2). In certain types of cancer such as melanoma, prostate cancer, and mesothelioma, integrin α7β1 is suggested to act as a tumor suppressor (9-11). Ren et al. [2007] isolated high and a low integrin α7-expressing cell lines from GBM, melanoma and prostate cancer and demonstrated that high integrin α7 expressing-cells show less invasiveness compared to low-integrin α7 expressing-cells. In the case of melanoma and prostate cancer, malignancy was abolished by forced expression of ITGA7 in the low-integrin α7 expressing cells (9). These opposing results may be explained by differences in the cells driving each type of cancer. Recent reports show that stem cell derived-gliomas display higher malignancy when compared to differentiating-cell derived tumors (12,21). Other studies show that prostate cancer growth can be mediated by luminal or basal differentiating-cells as reviewed in (22), while melanomas can be derived from both stem cell precursors or differentiating melanocytes depending on microenvironment (23,24). One possibility is that high-grade tumors are always derived from stem cell-like cells that express high levels of integrin α7 and influence the proliferative and invasive profile of these cells, whereas differentiated cell-derived tumors no longer express such high levels of integrin α7.
Tumor malignancy is also regulated by the microenvironment in which the tumor’s cell of origin is in contact with. Integrins are known to be primary transducers of microenvironmental cues (6), and therefore they are strong candidates to mediate the context dependent behavior of different tumorigenic cells of origin. Haas et al. [2017] analysis of glioma patient databases showed that the majority of GBM patients expressed high levels of integrin α3, integrin α6, and integrin α7. However, the co-expression of integrin α7 with integrin α3 and α6-integrins did not translate into a dramatically worse outcome when compared with patients only expressing high levels of integrin α7. This suggests that even though integrin α6 might play an important role in maintaining GSC identity (25), integrin α7 expression is strongly associated with tumor malignancy and its expression levels seem to be sufficient to determine the glioma patient prognosis. It is conceivable that the presence of different integrin combinations or stoichiometry in the cell surface might provide tissue type environmental cues that affect cell malignancy. Further studies are needed to elucidate the mechanism underlying the combined function of integrins during glioma malignancy and how integrin repertoires might influence tumor development.
One alternative non-exclusive hypothesis is that tumor malignancy is dependent on the integrin α7 isoform diversity. Alternative RNA splicing can generate isoforms α7A, α7B, and α7C with variations in the cytoplasmic domain, and isoforms α7X1 and α7X2 with distinct extracellular domains (7). These different isoforms display differing ligand specificities and therefore, differing distinct signaling outputs (7) which might affect tumorigenic cell behavior. Future studies should design experimental setups considering the integrin isoform diversity.
Haas et al. [2017] highlight a surprising function of integrin α7 in GBM malignancy. Together with previous papers, this study illustrates diverse context dependent-functions for integrin α7 in cancer. In addition, the authors provide a successful local therapy based on antibody injection into the brain that sets a solid framework for future cancer therapies. This advancement opens a new avenue for future studies on the nature of integrin α7-positive stem cell-derived tumors as targets therapeutic development.
Acknowledgements
Funding: This work was supported by grants from NIH/NIAMS (No. R01AR053697, R01AR064338) to DJ Burkin. P Barraza-Flores and CR Smith were supported by Mick Hitchcock Scholarship.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare
References
- Soomro SH, Ting LR, Qing YY, et al. Molecular biology of glioblastoma: Classification and mutational locations. J Pak Med Assoc 2017;67:1410-4. [PubMed]
- Singh SK, Clarke ID, Hide T, et al. Cancer stem cells in nervous system tumors. Oncogene 2004;23:7267-73. [Crossref] [PubMed]
- Ricci-Vitiani L, Pallini R, Biffoni M, et al. Tumour vascularization via endothelial differentiation of glioblastoma stem-like cells. Nature 2010;468:824-8. [Crossref] [PubMed]
- Cheng L, Huang Z, Zhou W, et al. Glioblastoma stem cells generate vascular pericytes to support vessel function and tumor growth. Cell 2013;153:139-52. [Crossref] [PubMed]
- Haas TL, Sciuto MR, Brunetto L, et al. Integrin alpha7 Is a Functional Marker and Potential Therapeutic Target in Glioblastoma. Cell Stem Cell 2017;21:35-50.e9. [Crossref] [PubMed]
- Barczyk M, Carracedo S, Gullberg D. Integrins. Cell Tissue Res 2010;339:269-80. [Crossref] [PubMed]
- Burkin DJ, Kaufman SJ. The alpha7beta1 integrin in muscle development and disease. Cell Tissue Res 1999;296:183-90. [Crossref] [PubMed]
- Hayashi YK, Chou FL, Engvall E, et al. Mutations in the integrin alpha7 gene cause congenital myopathy. Nat Genet 1998;19:94-7. [Crossref] [PubMed]
- Ren B, Yu YP, Tseng GC, et al. Analysis of integrin alpha7 mutations in prostate cancer, liver cancer, glioblastoma multiforme, and leiomyosarcoma. J Natl Cancer Inst 2007;99:868-80. [Crossref] [PubMed]
- Zhu ZH, Yu YP, Zheng ZL, et al. Integrin alpha 7 interacts with high temperature requirement A2 (HtrA2) to induce prostate cancer cell death. Am J Pathol 2010;177:1176-86. [Crossref] [PubMed]
- Laszlo V, Hoda MA, Garay T, et al. Epigenetic down-regulation of integrin alpha7 increases migratory potential and confers poor prognosis in malignant pleural mesothelioma. J Pathol 2015;237:203-14. [Crossref] [PubMed]
- Jiang Y, Marinescu VD, Xie Y, et al. Glioblastoma Cell Malignancy and Drug Sensitivity Are Affected by the Cell of Origin. Cell Rep 2017;19:1080-1. [Crossref] [PubMed]
- Collado M, Medema RH, Garcia-Cao I, et al. Inhibition of the phosphoinositide 3-kinase pathway induces a senescence-like arrest mediated by p27Kip1. J Biol Chem 2000;275:21960-8. [Crossref] [PubMed]
- Shtivelman E, Sussman J, Stokoe D. A role for PI 3-kinase and PKB activity in the G2/M phase of the cell cycle. Curr Biol 2002;12:919-24. [Crossref] [PubMed]
- Gu JJ, Fan KC, Zhang JH, et al. Suppression of microRNA-130b inhibits glioma cell proliferation and invasion, and induces apoptosis by PTEN/AKT signaling. Int J Mol Med 2018;41:284-92. [PubMed]
- Harburger DS, Calderwood DA. Integrin signalling at a glance. J Cell Sci 2009;122:159-63. [Crossref] [PubMed]
- Ming XY, Fu L, Zhang LY, et al. Integrin alpha7 is a functional cancer stem cell surface marker in oesophageal squamous cell carcinoma. Nat Commun 2016;7:13568. [Crossref] [PubMed]
- Dumont NA, Bentzinger CF, Sincennes MC, et al. Satellite Cells and Skeletal Muscle Regeneration. Compr Physiol 2015;5:1027-59. [Crossref] [PubMed]
- Yin H, Price F, Rudnicki MA. Satellite cells and the muscle stem cell niche. Physiol Rev 2013;93:23-67. [Crossref] [PubMed]
- Vachon PH, Xu H, Liu L, et al. Integrins (alpha7beta1) in muscle function and survival. Disrupted expression in merosin-deficient congenital muscular dystrophy. J Clin Invest 1997;100:1870-81. [Crossref] [PubMed]
- Liu C, Sage JC, Miller MR, et al. Mosaic analysis with double markers reveals tumor cell of origin in glioma. Cell 2011;146:209-21. [Crossref] [PubMed]
- Lee SH, Shen MM. Cell types of origin for prostate cancer. Curr Opin Cell Biol 2015;37:35-41. [Crossref] [PubMed]
- Kohler C, Nittner D, Rambow F, et al. Mouse Cutaneous Melanoma Induced by Mutant BRaf Arises from Expansion and Dedifferentiation of Mature Pigmented Melanocytes. Cell Stem Cell 2017;21:679-93.e6. [Crossref] [PubMed]
- Moon H, Donahue LR, Choi E, et al. Melanocyte Stem Cell Activation and Translocation Initiate Cutaneous Melanoma in Response to UV Exposure. Cell Stem Cell 2017;21:665-78.e6. [Crossref] [PubMed]
- Lathia JD, Gallagher J, Heddleston JM, et al. Integrin alpha 6 regulates glioblastoma stem cells. Cell Stem Cell 2010;6:421-32. [Crossref] [PubMed]
Cite this article as: Nunes AM, Barraza-Flores P, Smith CR, Burkin DJ. Integrin α7: a major driver and therapeutic target for glioblastoma malignancy. Stem Cell Investig 2017;4:97.


