In the heart of the in vivo reprogramming
Myocardial infarction (MI) is caused by the occlusion of coronary arteries in the heart leading to an oxygen supply-demand imbalance in the myocardium, eventually causing ischemia of the perfused tissue that—if prolonged—leads to myocardial cell death. The diagnosis of acute MI is made when the acute myocardial injury is associated with a rise and/or fall of cardiac troponin values above the 99th percentile upper reference limit. In addition, at least one of MI related findings—including clinical symptoms, new ECG changes related to the ischemia, development of pathological Q waves, imaging evidence of new loss of viable myocardium and motion abnormality consistent with ischemia—need to be present (1). Symptoms associated with MI are non-specific and include dyspnea, fatigue, discomfort of chest and upper extremities, and mandibular or epigastric pain either during exertion or at rest. According to WHO, MI and cardiovascular diseases are the single leading cause of mortality, accounting for 30% of all global deaths. The rates of death by MI have decreased in western societies between 1990 and 2010 thanks to improved reperfusion in the acute setting as well as preventive lifestyle measures and improved therapeutic regimens including long-term treatment with aspirin, beta-blockers, and statins. However, there are currently no treatments to avoid the consequences of nonfunctional connective tissue deposition secondary to MI, including heart failure, cardiogenic shock, and cardiac arrest. Several approaches using stem cell-based therapies have been considered in MI and the use of adult stem cells including mesenchymal and cardiac stem cells resulted in modest effects on cardiac recovery. Although promising, the use of derivatives from pluripotent stem cells to drive their fate toward a cardiovascular lineage retains the risk to generate a teratoma from residual pluripotent cells that have not reacted to the lineage-specific instructive cues. Thus, therapies aimed at replacing the connective tissue in the heart to avoid these collateral deaths would be of extreme value for a large portion of these patients.
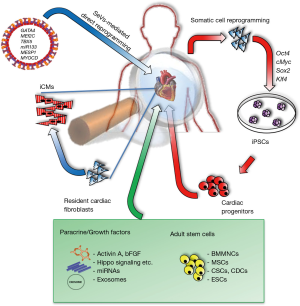
In the Issue 1, Volume 22 of Cell Stem Cell, Miyamoto and colleagues (2) showed that non-integrating Sendai virus (SeV) vectors expressing cardiac-lineage transcription factors Gata4, Mef2c, and Tbx5 (GMT) reprogrammed mouse and human fibroblasts into induced cardiomyocyte-like cells (iCMs) without going through the cardiac progenitor state. Ieda et al. reported that reprogramming of endogenous or explanted fibroblasts might provide a source of cardiomyocytes for regenerative approaches in 2010 (3). After 4 years he demonstrated that the addition of miR-133a (miR-133) to GMT plus Mesp1 and Myocd improved cardiac reprogramming from mouse or human fibroblasts (4). Compared to previous papers (3-5), the new approach of generating iCMs using SeV was proved to be extremely efficient in reprogramming resident cardiac fibroblasts into iCMs. Lineage tracing studies on reprogrammed local fibroblasts show that these newly generated cardiomyocytes (CMs) are not originated by fusion events with local CMs but they are rather raised directly from fibroblasts (5). This was similarly shown in this SeV-mediated generation of iCMs described by Miyamoto and colleagues, where in vivo lineage tracing showed reduced fibrosis, improved cardiac function, and demonstrated the effects of stem cell derivatives on cardiac remodeling in MI animal model.
Depending on the downstream applications, different types of reprogramming strategies (Figure 1) can be adopted considering the desired balance among reprogramming efficiency, residual transgene retention, aneuploidy, costs and workload. To date, direct reprogramming of fibroblasts into CMs has been realized using retroviruses that insert copy of their transgenes into the DNA of infected cells. This can cause insertional mutagenesis, disturbing endogenous gene expression and might lead to cancer. The non-integrating properties of SeV, on the other hand, make the use of this technology more intriguing for a potential use in the clinic. Moreover, SeVs exhibit higher transduction efficiencies and maintain higher transgene expression patterns as reported by Ieda’s group. Of note, using this approach it is possible to abolish fibroblast signatures, including their expansion ability, extracellular matrix synthesis as well as their cytokine secretome.
However interesting, the paper in Issue 1, Volume 22 of Cell Stem Cell lacks mechanistic insights on the drivers responsible for SeV-mediated fibroblast conversion into CMs. The same group reported in Issue 3, Volume 23 of Cell Stem Cell, a study regarding direct reprogramming-based screening of genes involved in nascent mesoderm induction (6). Although the authors showed that Tbx6 is critical for mesoderm induction and subsequent lineage diversification, it is not clear if Tbx6 is a crucial mediator of direct reprogramming from fibroblast into CMs.
Besides direct reprogramming, other strategies are investigated to counteract MI consequences. Non-cardiac cells, including skeletal myoblasts, bone marrow mononuclear cells (BMMNCs) and mesenchymal stem cells (MSCs), have been tested in cell-based approaches to treat cardiac diseases. Firstly, satellite cells, mainly involved in skeletal muscle regeneration were used to remuscularize the postinfarction scars but failed to improve heart function (7). Next, the use of BMMNCs in MI animal models (8) and clinical trials, like BOOST trial and REPAIR-AMI trial, showed beneficial effects compared to controls. Unfortunately, earlier results could not be reproduced in several randomized, double-blinded clinical trials including larger patient populations (9). In addition, in clinical trials, like POSEIDON and MSC-HF, injected MSCs contributed only to modest benefits after an ischemic event (10,11). Doubts arose according to the cardiomyogenic potential of MSCs and BMMNCs. Therefore, the newly identified cardiac stem cells (CSCs) and cardiosphere-derived cells (CDCs) gained momentum for replacing lost tissue in the damaged heart. However, clinical trials, such as SCIPIO and CADUCEUS showed slightly positive effects in LVEF or infarct size in patients treated with CSC or cardiosphere-derived cells (CDCs) therapies (12,13). Several research groups have documented the use of three-dimensional in vitro engineered scaffolds or biomaterials to deliver cells into the myocardium and to attract cells from endogenous healing or to support the ventricle wall, maintaining its geometry during remodeling. Approaches, in which induced pluripotent stem cells (iPSCs) embedded in matrix-enriched hydrogels, have been reported to improve therapeutic effects after MI. Two main questions arose from the use of iPSCs to treat cardiac dysfunction: (I) the teratogenic potential of iPSCs that do not properly differentiate into adult cells (II) the engraftment efficiency of iPSC derivatives. Nevertheless, in recent years the direct use of embryonic stem cell-derived cardiac progenitors in the treatment of severe heart failure has been reported in patients (14).
An interesting strategy that could avoid these risks is to transdifferentiate local cardiac fibroblasts, directly into de novo functional CMs. Moreover, a huge fibrotic response occurs after MI and plays a major role in contributing to the dysfunctional state seen in advanced cardiomyopathy. Thus, transdifferentiating cardiac fibroblasts into CMs is a highly appealing approach to repair the damaged heart and to diminish post-MI fibrotic tissues. Interestingly, the iCMs generated with Ieda's strategy showed similar properties to endogenous CMs including, gene expression profiles, ultrastructural organization, and contractility due to the correct sarcomeric calcium gradients. SeV-GMT infection resulted in the generation of the three subtypes of functional iCMs (i.e., atrial, ventricular and pacemaker) as documented by electrophysiological data. However, iCMs characterized by atrial-like action potentials (APs) were the most abundant, suggesting a more immature phenotype. Although it is impossible to evaluate the electrical characteristics of integrated iCMs, it is likely that local growth factors could be involved in their final maturation. Nevertheless, we cannot exclude the presence of atrial cardiomyocytes in post-MI GMT-regenerated ventricles that could affect cardiac function.
Often a mixture of in vitro-generated CMs, exhibiting atrial-, ventricular- or nodal-like APs may obstruct desired outcome or complicate further analysis. Strategies to guide CM subtype fating involve stage-specific regulation of various signaling molecules. Basic fibroblast growth factor (bFGF), transforming growth factor beta (TGF-β)/activin A/NODAL, bone morphogenetic protein (BMP), Wnt and Hippo signaling cascade (YAP/TAZ) signaling pathways have been shown to play a key role in cardiomyogenesis, while the exogenous addition of retinoic acid (RA) drives towards atrial cell commitment (15,16). Thus, further studies are necessary to better define an ad hoc protocol for the generation of specific CMs.
Paradoxically, transplanted cardiomyogenic stem cells rarely differentiated into CMs, suggesting that paracrine effects could be responsible for the minor beneficial effects observed. On the other hand, stem cell secreted-exosomes, extracellular vesicles with a reported diameter up to 100nm may provide an alternative therapeutic approach for cardiac repair. Indeed, the cargo of mRNA, proteins, and miRNA in exosomes might be crucial for cardiac repair and regeneration. For example, the administration of CDC-derived exosomes inhibits CM apoptosis and the use of extracellular vesicles, derived from human CSCs, reduced heart remodeling and ameliorated cardiac function in MI murine models (17,18). Since these therapeutic approaches are tackling different aspects of cardiac degeneration, they can be combined with gene therapies as previously reported by Ieda’s group (4) to achieve synergistic effects.
The novel findings in the report by Ieda’s group have highlighted the potential of an integration-free method based on SeV vectors carrying on specific GMT transcription factors and able to induce fibroblast transdifferentiation into functional iCMs. Moreover, SeV vectors are also effective in murine models, improving fibroblast reprogramming, diminishing scar tissue formation post-MI. The observed anti-fibrotic effects could be potentially highly interesting for therapeutic applications for chronic cardiac diseases, where the aberrant accumulation of connective tissue is highly present. The fact that SeV particles in vivo infect preferentially non-myocytes, mainly cardiac fibroblasts is also of interest. This preferential selection could be explained due to the presence of hemagglutinin-neuraminidase (HN) proteins at the external viral lipid bilayer. Although the precise mechanisms still have to be elucidated, SeV HN-mediated reprogramming strategies may be considered as specific delivery system for fibroblasts implicated in many degenerative diseases including, among others, pulmonary fibrosis, cystic fibrosis, glial scar, cirrhosis and arterial stiffness.
In literature there are examples where in vivo reprogramming is efficiently used in other diseases that may require replacement of lost tissue in poorly regenerating organs. One of the most interesting fields where the use of direct reprogramming has unlimited applications is the neurological one. In 2013 a paper by Arlotta’s group described the reprogramming between two different excitatory neuron subtypes in the neocortex (i.e., from callosal to corticofugal projection neurons) within the brain itself (19). The reprogramming was demonstrated to be efficiently occurring during a temporal window of embryonic and early postnatal development, but nevertheless showed the ability to change fate specification even post-mitotically. The following year, Guo et al. have applied this type of technology to two different brain disease models. In their paper they showed that retroviral expression of NeuroD1 in astrocytes efficiently reprogrammed the cells into functional glutamatergic neurons. Following the validation in vitro, it was shown that the newly reprogrammed cells efficiently integrated into and contributed to the neural circuits of the brains of both stab-injured and Alzheimer’s disease models (20). However, both these papers have used integrating viruses to reprogram neurons, which is not clinically translatable as the random integration by the retrovirus in the cell genome may have potentially tumorigenic consequences. To this end, last year the use of non-viral approaches for in vivo reprogramming has been investigated for the in vivo lineage conversion of fibroblasts into neurons in mouse Parkinson’s disease model (21). It was previously demonstrated that electromagnetic fields have significant effects on several cellular processes including the forced expression of transcription factors, thus paving the way to the use of this promotion of cell-fate conversion in regenerative medicine. In their paper, Yoo et al. transferred the electromagnetic field frequency to fibroblasts using gold nanoparticles to enhance the efficacy of direct lineage reprogramming into induced dopaminergic neurons in the presence of transient reprogramming transcription-factor expression in vitro. Intriguingly, they have been able to have a higher conversion rates from somatic cells into induced dopaminergic neurons compared to previous studies, and the in vivo neurons exhibit midbrain dopaminergic neuron characteristics.
In conclusion, the use of direct reprogramming mediated by SeV revealed a high efficiency of cell conversion and the possibility to be applied in different pathologies makes this technology of broad interest. Understanding the potential side effects is the first step in the development of new treatment strategies and this required further studies to ensure that aberrant differentiation of fibroblasts do not occur in functional connective tissues. Thus, the interesting data by Miyamoto et al. published in Cell Stem Cell solicit focused follow up comparative studies to better understand the mechanisms of SeV-mediated transduction and possible alternative ways to improve safety in direct cell-conversion technologies.
Acknowledgements
Funding: Fonds Wetenschappelijk Onderzoek (#G088715N, #G060612N, #G0A8813N, #1S90718N). CARIPLO Foundation #2015_0634. IUAP-VII/07 #EJJ-C4851-17/07-P. KU Leuven - Project Financiering Stem Cells #ETH-C1900-PF. We would also like to thank Rondoufonds voor Duchenne Onderzoek for a kind donation.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Thygesen K, Alpert JS, Jaffe AS, et al. Fourth Universal Definition of Myocardial Infarction. J Am Coll Cardiol 2018. [Epub ahead of print]. [PubMed]
- Miyamoto K, Akiyama M, Tamura F, et al. Direct In Vivo Reprogramming with Sendai Virus Vectors Improves Cardiac Function after Myocardial Infarction. Cell Stem Cell 2018;22:91-103.e5. [Crossref] [PubMed]
- Ieda M, Fu JD, Delgado-Olguin P, et al. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell 2010;142:375-86. [Crossref] [PubMed]
- Muraoka N, Yamakawa H, Miyamoto K, et al. MiR-133 promotes cardiac reprogramming by directly repressing Snai1 and silencing fibroblast signatures. EMBO J 2014;33:1565-81. [Crossref] [PubMed]
- Qian L, Huang Y, Spencer CI, et al. In vivo reprogramming of murine cardiac fibroblasts into induced cardiomyocytes. Nature 2012;485:593-8. [Crossref] [PubMed]
- Sadahiro T, Isomi M, Muraoka N, et al. Tbx6 Induces Nascent Mesoderm from Pluripotent Stem Cells and Temporally Controls Cardiac versus Somite Lineage Diversification. Cell Stem Cell 2018;23:382-95.e5. [Crossref] [PubMed]
- Menasché P, Alfieri O, Janssens S, et al. The Myoblast Autologous Grafting in Ischemic Cardiomyopathy (MAGIC) trial: first randomized placebo-controlled study of myoblast transplantation. Circulation 2008;117:1189-200. [Crossref] [PubMed]
- Orlic D, Kajstura J, Chimenti S, et al. Bone marrow cells regenerate infarcted myocardium. Nature 2001;410:701-5. [Crossref] [PubMed]
- Sürder D, Manka R, Lo Cicero V, et al. Intracoronary injection of bone marrow-derived mononuclear cells early or late after acute myocardial infarction: effects on global left ventricular function. Circulation 2013;127:1968-79. [Crossref] [PubMed]
- Hare JM, Fishman JE, Gerstenblith G, et al. Comparison of allogeneic vs autologous bone marrow–derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: the POSEIDON randomized trial. JAMA 2012;308:2369-79. [Crossref] [PubMed]
- Mathiasen AB, Qayyum AA, Jørgensen E, et al. Bone marrow-derived mesenchymal stromal cell treatment in patients with severe ischaemic heart failure: a randomized placebo-controlled trial (MSC-HF trial). Eur Heart J 2015;36:1744-53. [Crossref] [PubMed]
- Bolli R, Chugh AR, D'Amario D, et al. Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomised phase 1 trial. Lancet 2011;378:1847-57. [Crossref] [PubMed]
- Makkar RR, Smith RR, Cheng K, et al. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial. Lancet 2012;379:895-904. [Crossref] [PubMed]
- Menasché P, Vanneaux V, Hagège A, et al. Transplantation of Human Embryonic Stem Cell-Derived Cardiovascular Progenitors for Severe Ischemic Left Ventricular Dysfunction. J Am Coll Cardiol 2018;71:429-38. [Crossref] [PubMed]
- Duelen R, Gilbert G, Patel A, et al. Activin A Modulates CRIPTO-1/HNF4α+ Cells to Guide Cardiac Differentiation from Human Embryonic Stem Cells. Stem Cells Int 2017;2017. [Crossref] [PubMed]
- Xin M, Kim Y, Sutherland LB, et al. Hippo pathway effector Yap promotes cardiac regeneration. Proc Natl Acad Sci U S A 2013;110:13839-44. [Crossref] [PubMed]
- Barile L, Lionetti V, Cervio E, et al. Extracellular vesicles from human cardiac progenitor cells inhibit cardiomyocyte apoptosis and improve cardiac function after myocardial infarction. Cardiovasc Res 2014;103:530-41. [Crossref] [PubMed]
- Gallet R, Dawkins J, Valle J, et al. Exosomes secreted by cardiosphere-derived cells reduce scarring, attenuate adverse remodelling, and improve function in acute and chronic porcine myocardial infarction. Eur Heart J 2017;38:201-11. [PubMed]
- Rouaux C, Arlotta P. Direct lineage reprogramming of postmitotic callosal neurons into corticofugal neurons in vivo. Nat Cell Biol 2013;15:214-221. [Crossref] [PubMed]
- Guo Z, Zhang L, Wu Z, et al. In vivo direct reprogramming of reactive glial cells into functional neurons after brain injury and in an Alzheimer's disease model. Cell Stem Cell 2014;14:188-202. [Crossref] [PubMed]
- Yoo J, Lee E, Kim HY, et al. Electromagnetized gold nanoparticles mediate direct lineage reprogramming into induced dopamine neurons in vivo for Parkinson's disease therapy. Nat Nanotechnol 2017;12:1006-14. [Crossref] [PubMed]
Cite this article as: Sampaolesi M, Pozzo E, Duelen R. In the heart of the in vivo reprogramming. Stem Cell Investig 2018;5:38.


