
Stem cell-based regenerative medicine
Definition and history of stem cell
Stem cells are one of the main cells of the human body that have ability to grow more than 200 types of body cells (1). Stem cells, as non-specialized cells, can be transformed into highly specialized cells in the body (2). In the other words, Stem cells are undifferentiated cells with self-renewal potential, differentiation into several types of cells and excessive proliferation (3). In the past, it was believed that stem cells can only differentiate into mature cells of the same organ. Today, there are many evidences to show that stem cells can differentiate into the other types of cell as well as ectoderm, mesoderm and endoderm. The numbers of stem cells are different in the tissues such as bone marrow, liver, heart, kidney, and etc. (3,4). Over the past 20 years, much attention has been paid to stem cell biology. Therefore, there was a profound increase in the understanding of its characteristics and the therapeutic potential for its application (5). Today, the utilization of these cells in experimental research and cell therapy represents in such disorders including hematological, skin regeneration and heart disease in both human and veterinary medicine (6).The history of stem cells dates back to the 1960s, when Friedenstein and colleagues isolated, cultured and differentiated to osteogenic cell lineage of bone marrow-derived cells from guinea pigs (7). This project created a new perspective on stem cell research. In the following, other researchers discovered that the bone marrow contains fibroblast-like cells with congenic potential in vitro, which were capable of forming colonies (CFU-F) (8). For over 60 years, transplantation of hematopoietic stem cells (HSCs) has been the major curative therapy for several genetic and hematological disorders (9). Almost in 1963, Till and McCulloch described a single progenitor cell type in the bone marrow which expand clonally and give rise to all lineages of hematopoietic cells. This research represented the first characterization of the HSCs (10). Also, the identification of mouse embryonic stem cells (ESCs) in 1981 revolutionized the study of developmental biology, and mice are now used extensively as one of the best option to study stem cell biology in mammals (11). Nevertheless, their application a model, have limitations in the regenerative medicine. But this model, relatively inexpensive and can be easily manipulated genetically (12). Failure to obtain a satisfactory result in the selection of many mouse models, to recapitulate particular human disease phenotypes, has forced researchers to investigate other animal species to be more probably predictive of humans (13). For this purpose, to study the genetic diseases, the pig has been currently determined as one the best option of a large animal model (14).
Classification of stem cells
Stem cells, based on their differentiation ability, are classified into different cell types, including totipotent, pluripotent, multipotent, or unipotent. Also, another classification of these cells are based on the evolutionary stages, including embryonic, fetal, infant or umbilical cord blood and adult stem cells (15). Figure 1 shows an overview of stem cells classifications based on differentiation potency.
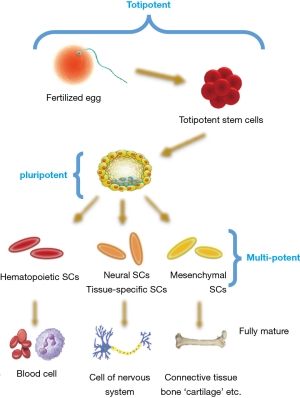
Toti-potent stem cells
Toti-potent cells have the potential for development to any type of cell found in the organism. In the other hand, the capacity of these cells to develop into the three primary germ cell layers of the embryo and into extra-embryonic tissues such as the placenta is remarkable (15).
Pluri-potent stem cells
The pluripotent stem cells are kind of stem cells with the potential for development to approximately all cell types. These cells contain ESCs and cells that are isolated from the mesoderm, endoderm and ectoderm germ layers that are organized in the beginning period of ESC differentiation (15).
Multi-potent stem cells
The multipotent stem cells have less proliferative potential than the previous two groups and have ability to produce a variety of cells which limited to a germinal layer [such as mesenchymal stem cells (MSCs)] or just a specific cell line (such as HSCs). Adult stem cells are also often in this group. In the word, these cells have the ability to differentiate into a closely related family of cells (15).
Uni-potent stem cells
Despite the increasing interest in totipotent and pluripotent stem cells, unipotent stem cells have not received the most attention in research. A unipotent stem cell is a cell that can create cells with only one lineage differentiation. Muscle stem cells are one of the example of this type of cell (15). The word ‘uni’ is derivative from the Latin word ‘unus’ meaning one. In adult tissues in comparison with other types of stem cells, these cells have the lowest differentiation potential. The unipotent stem cells could create one cell type, in the other word, these cells do not have the self-renewal property. Furthermore, despite their limited differentiation potential, these cells are still candidates for treatment of various diseases (16).
ESCs
ESCs are self-renewing cells that derived from the inner cell mass of a blastocyst and give rise to all cells during human development. It is mentioned that these cells, including human embryonic cells, could be used as suitable, promising source for cell transplantation and regenerative medicine because of their unique ability to give rise to all somatic cell lineages (17). In the other words, ESCs, pluripotent cells that can differentiate to form the specialized of the various cell types of the body (18). Also, ESCs capture the imagination because they are immortal and have an almost unlimited developmental potential. Due to the ethical limitation on embryo sampling and culture, these cells are used less in research (19).
HSCs
HSCs are multipotent cells that give rise to blood cells through the process of hematopoiesis (20). These cells reside in the bone marrow and replenish all adult hematopoietic lineages throughout the lifetime of the human and animal (21). Also, these cells can replenish missing or damaged components of the hematopoietic and immunologic system and can withstand freezing for many years (22).The mammalian hematopoietic system containing more than ten different mature cell types that HSCs are one of the most important members of this. The ability to self-renew and multi-potency is another specific feature of these cells (23).
Tissue specific stem cell or adult stem cells
Adult stem cells, as undifferentiated cells, are found in numerous tissues of the body after embryonic development. These cells multiple by cell division to regenerate damaged tissues (24). Recent studies have been shown that adult stem cells may have the ability to differentiate into cell types from various germ layers. For example, bone marrow stem cells which is derived from mesoderm, can differentiate into cell lineage derived mesoderm and endoderm such as into lung, liver, GI tract, skin, etc. (25). Another example of adult stem cells is neural stem cells (NSCs), which is derived from ectoderm and can be differentiate into another lineage such as mesoderm and endoderm (26). Therapeutic potential of adult stem cells in cell therapy and regenerative medicine has been proven (27).
Cancer stem cells (CSCs)
For the first time in the late 1990s, CSCs were identified by John Dick in acute myeloid diseases. CSCs are cancerous cells that found within tumors or hematological cancers. Also, these cells have the characteristics of normal stem cells and can also give rise to all cell types found in a particular cancer sample (28). There is an increasing evidence supporting the CSCs hypothesis. Normal stem cells in an adult living creature are responsible for the repair and regeneration of damaged as well as aged tissues (29). Many investigations have reported that the capability of a tumor to propagate and proliferate relies on a small cellular subpopulation characterized by stem-like properties, named CSCs (30).
MSCs
Embryonic connective tissue contains so-called mesenchymes, from which with very close interactions of endoderm and ectoderm all other connective and hematopoietic tissues originate, Whereas, MSCs do not differentiate into hematopoietic cell (31). In 1924, Alexander A. Maxi mow used comprehensive histological detection to identify a singular type of precursor cell within mesenchyme that develops into various types of blood cells (32). In general, MSCs are type of cells with potential of multi-lineage differentiation and self-renewal, which exist in many different kinds of tissues and organs such as adipose tissue, bone marrow, skin, peripheral blood, fallopian tube, cord blood, liver and lung et al. (4,5). Today, stem cells are used for different applications. In addition to using these cells in human therapy such as cell transplantation, cell engraftment etc. The use of stem cells in veterinary medicine has also been considered. The purpose of this review is to provide a summary of our current knowledge regarding the important and types of isolated stem cells from different sources of animal models such as horse, pig, goat, dog, rabbit, cat, rat, mice etc. In this regard, due to the widespread use and lot of attention of MSCs, in this review, we will elaborate on use of MSCs in veterinary medicine.
Isolation, culture and identification of MSCs
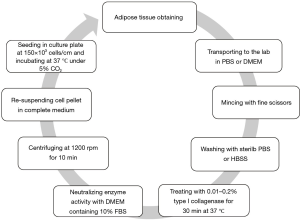
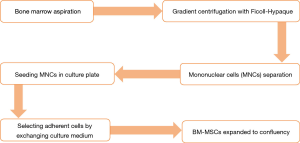
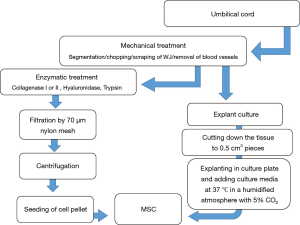
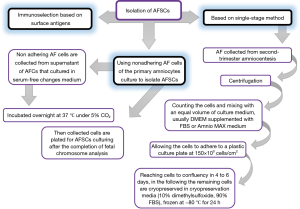
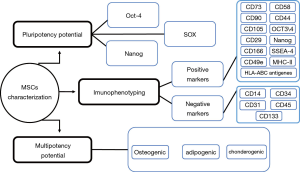
The isolation method, maintenance and culture condition of MSCs differs from the different tissues, these methods as well as characterization of MSCs described as Figures 2-6 (36). MSCs could be isolated from the various tissues such as adipose tissue, bone marrow, umbilical cord, amniotic fluid etc. (37).
Application of MSCs in regenerative medicine in animal models
The diversity of stem cell or MSCs sources and a wide aspect of potential applications of these cells cause to challenge for selecting an appropriate cell type for cell therapy (38). Various diseases in animals have been treated by cell-based therapy. However, there are immunity concerns regarding cell therapy using stem cells. Improving animal models and selecting suitable methods for engraftment and transplantation could help address these subjects, facilitating eventual use of stem cells in the clinic. Therefore, for this purpose, in this section of this review, we provide an overview of the current as well as previous studies for future development of animal models to facilitate the utilization of stem cells in regenerative medicine (14). Significant progress has been made in stem cells-based regenerative medicine, which enables researchers to treat those diseases which cannot be cured by conventional medicines. The unlimited self-renewal and multi-lineage differentiation potential to other types of cells causes stem cells to be frontier in regenerative medicine (24). More researches in regenerative medicine have been focused on human cells including embryonic as well as adult stem cells or maybe somatic cells. Today there are versions of embryo-derived stem cells that have been reprogrammed from adult cells under the title of “pluripotent cells” (39). Stem cell therapy has been developed in the last decade. Nevertheless, obstacles including unwanted side effects due to the migration of transplanted cells as well as poor cell survival have remained unresolved. In order to overcome these problems, cell therapy has been introduced using biocompatible and biodegradable biomaterials to reduce cell loss and long-term in vitro retention of stem cells.
Currently in clinical trials, these biomaterials are widely used in drug and cell-delivery systems, regenerative medicine and tissue engineering in which to prevent the long-term survival of foreign substances in the body the release of cells are controlled (40).
Heart failure
Today, the incidence and prevalence of heart failure in human societies is a major and increasing problem that unfortunately has a poor prognosis. For decades, MSCs have been used for cardiovascular regenerative therapy as one of the potential therapeutic agents (41). Dhein et al. [2006] found that autologous bone marrow-derived mesenchymal stem cells (BMSCs) transplantation improves cardiac function in non-ischemic cardiomyopathy in a rabbit model. In one study, Davies et al. [2010] reported that transplantation of cord blood stem cells in ovine model of heart failure, enhanced the function of heart through improvement of right ventricular mass, both systolic and diastolic right heart function (42). In another study, Nagaya et al. [2005] found that MSCs dilated cardiomyopathy (DCM), possibly by inducing angiogenesis and preventing cardial fibrosis. MSCs have a tremendous beneficial effect in cell transplantation including in differentiating cardiomyocytes, vascular endothelial cells, and providing anti-apoptotic as well angiogenic mediators (43). Roura et al. [2015] shown that umbilical cord blood mesenchymal stem cells (UCBMSCs) are envisioned as attractive therapeutic candidates against human disorders progressing with vascular deficit (44). Ammar et al., [2015] compared BMSCs with adipose tissue-derived MSCs (ADSCs). It was demonstrated that both BMSCs and ADSCs were equally effective in mitigating doxorubicin-induced cardiac dysfunction through decreasing collagen deposition and promoting angiogenesis (45).
Small mammalian animal models for heart disease
There are many advantages of small animal models usage in cardiovascular research compared with large animal models. Small model of animals has a short life span, which allow the researchers to follow the natural history of the disease at an accelerated pace. Some advantages and disadvantages are listed in Table 1 (46).
Large mammalian animal models for heart disease
Despite of the small animal model, large animal models are suitable models for studies of human diseases. Some advantages and disadvantages of using large animal models in a study protocol planning was elaborated in Table 2 (47).
Wound healing
Chronic wound is one of the most common problem and causes significant distress to patients (48). Among the types of tissues that stem cells derived it, dental tissue–derived MSCs provide good sources of cytokines and growth factors that promote wound healing. The results of previous studies showed that stem cells derived deciduous teeth of the horse might be a novel approach for wound care and might be applied in clinical treatment of non-healing wounds (49). However, the treatment with stem cells derived deciduous teeth needs more research to understand the underlying mechanisms of effective growth factors which contribute to the wound healing processes (50). This preliminary investigation suggests that deciduous teeth-derived stem cells have the potential to promote wound healing in rabbit excisional wound models (49). In the another study, Lin et al. [2013] worked on the mouse animal model and showed that ADSCs present a potentially viable matrix for full-thickness defect wound healing (51).
Tooth regeneration
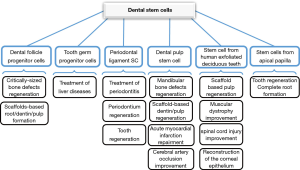
Many studies have been done on dental reconstruction with MSCs. In one study, Khorsand et al. [2013] reported that dental pulp-derived stem cells (DPSCs) could promote periodontal regeneration in canine model. Also, it was shown that canine DPSCs were successfully isolated and had the rapid proliferation and multi-lineage differentiation capacity (52). Other application of dental-derived stem cells is shown in Figure 7.
Application of MSCs in neurodegenerative disease in animal model
As noted above, stem cells have different therapeutic applications and self-renewal capability. These cells can also differentiate into the different cell types. There is now a great hope that stem cells can be used to treat diseases such as Alzheimer, Parkinson and other serious diseases. In stem cell-based therapy, ESCs are essentially targeted to differentiate into functional neural cells. Today, a specific category of stem cells called induced pluripotent stem (iPS) cells are being used and tested to generate functional dopamine neurons for treating Parkinson's disease of a rat animal model. In addition, NSC as well as MSCs are being used in neurodegenerative disorder therapies for Alzheimer’s disease, Parkinson’s disease, and stroke (54). Previous studies have shown that BMSCs could reduce brain amyloid deposition and accelerate the activation of microglia in an acutely induced Alzheimer’s disease in mouse animal model. Lee et al. [2009] reported that BMSCs can increase the number of activated microglia, which effective therapeutic vehicle to reduce Aβ deposits in AD patients (55). In confirmation of previous study, Liu et al. [2015] showed that transplantation of BMSCs in brain of mouse model of Alzheimer’s disease cause to decrease in amyloid beta deposition, increase in brain-derived neurotrophic factor (BDNF) levels and improvements in social recognition (56). In addition of BMSCs, NSCs have been proposed as tools for treating neurodegeneration disease because of their capability to create an appropriate cell types which transplanted. Åkerud et al. [2001] demonstrated that NSCs efficiently express high level of glial cell line-derived neurotrophic factor (GDNF) in vivo, suggesting a use of these cells in the treatment of neurodegenerative disorders, including Parkinson’s disease (57). In the following, Venkataramana et al. [2010] transplanted BMSCs into the sub lateral ventricular zones of seven Parkinson’s disease patients and reported encouraging results (58).
Conclusions
The human body is fortified with specialized cells named MSCs, which has the ability to self-renew and differentiate into various cell types including, adipocyte, osteocyte, chondrocyte, neurons etc. In addition to mentioned properties, these cells can be easily isolated, safely transplanted to injured sites and have the immune regulatory properties. Numerous in vitro and in vivo studies in animal models have successfully demonstrated the potential of MSCs for various diseases; however, the clinical outcomes are not very encouraging. Based on the studies in the field of stem cells, MSCs find wide application in treatment of diseases, such as heart failure, wound healing, tooth regeneration and etc. In addition, these cells are particularly important in the treatment of the sub-branch neurodegenerative diseases like Alzheimer and Parkinson.
Acknowledgments
The authors wish to thank staff of the Stem Cell Research Center, Tabriz University of Medical Sciences, Tabriz, Iran.
Funding: The project described was supported by Grant Number “IR.TBZMED.REC.1396.1218” from the Stem Cell Research Center, Tabriz University of Medical Sciences, Tabriz, Iran.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Amit M, Shariki C, Margulets V, et al. Feeder layer-and serum-free culture of human embryonic stem cells. Biol Reprod 2004;70:837-45. [Crossref] [PubMed]
- Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007;131:861-72. [Crossref] [PubMed]
- Fathi E, Farahzadi R. Isolation, culturing, characterization and aging of adipose tissue-derived mesenchymal stem cells: a brief overview. Brazilian Archives of Biology and Technology 2016.59.
- Ejtehadifar M, Shamsasenjan K, Movassaghpour A, et al. The Effect of Hypoxia on Mesenchymal Stem Cell Biology. Adv Pharm Bull 2015;5:141-9. [Crossref] [PubMed]
- Mohammadian M, Shamsasenjan K, Lotfi Nezhad P, et al. Mesenchymal stem cells: new aspect in cell-based regenerative therapy. Adv Pharm Bull 2013;3:433-7. [PubMed]
- Markoski MM. Advances in the use of stem cells in veterinary medicine: from basic research to clinical practice. Scientifica (Cairo) 2016;2016:4516920. [Crossref] [PubMed]
- Friedenstein AJ, Chailakhyan RK, Latsinik NV, et al. Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues: cloning in vitro and retransplantation in vivo. Transplantation 1974;17:331-40. [Crossref] [PubMed]
- Banas A, Teratani T, Yamamoto Y, et al. Adipose tissue-derived mesenchymal stem cells as a source of human hepatocytes. Hepatology 2007;46:219-28. [Crossref] [PubMed]
- Ghimire S, Weber D, Mavin E, et al. Pathophysiology of gvhd and other hsct-related major complications. Front Immunol 2017;8:79. [Crossref] [PubMed]
- Becker AJ, McCulloch EA, Till JE. Cytological demonstration of the clonal nature of spleen colonies derived from transplanted mouse marrow cells. Nature 1963;197:452-4. [Crossref] [PubMed]
- Lotfinegad P, Shamsasenjan K, Movassaghpour A, et al. Immunomodulatory nature and site specific affinity of mesenchymal stem cells: a hope in cell therapy. Adv Pharm Bull 2014;4:5-13. [PubMed]
- Song J, Yang D, Ruan J, et al. Production of immunodeficient rabbits by multiplex embryo transfer and multiplex gene targeting. Sci Rep 2017;7:12202. [Crossref] [PubMed]
- Harding J, Roberts RM, Mirochnitchenko O. Large animal models for stem cell therapy. Stem Cell Res Ther 2013;4:23. [Crossref] [PubMed]
- Cibelli J, Emborg ME, Prockop DJ, et al. Strategies for improving animal models for regenerative medicine. Cell Stem Cell 2013;12:271-4. [Crossref] [PubMed]
- Kalra K, Tomar P. Stem Cell: Basics, Classification and Applications. American Journal of Phytomedicine and Clinical Therapeutics 2014;27:919-30.
- Kote Amol P, Pawar Sanjay D, Dhonde Satish M, et al. An overview of stem cell. Pharmacologyonline 2011;3:1155-70.
- Levenberg S, Huang NF, Lavik E, et al. Differentiation of human embryonic stem cells on three-dimensional polymer scaffolds. Proc Natl Acad Sci U S A 2003;100:12741-6. [Crossref] [PubMed]
- Lu S-J, Li F, Vida L, et al. CD34+ CD38-hematopoietic precursors derived from human embryonic stem cells exhibit an embryonic gene expression pattern. Blood 2004;103:4134-41. [Crossref] [PubMed]
- Rippon HJ, Bishop AE. Embryonic stem cells. Cell Prolif 2004;37:23-34. [Crossref] [PubMed]
- Birbrair A, Frenette PS. Niche heterogeneity in the bone marrow. Ann N Y Acad Sci 2016;1370:82-96. [Crossref] [PubMed]
- Goodell MA, Brose K, Paradis G, et al. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med 1996;183:1797-806. [Crossref] [PubMed]
- Trigg ME. Hematopoietic stem cells. Pediatrics 2004;113:1051-7. [PubMed]
- Seita J, Weissman IL. Hematopoietic stem cell: self-renewal versus differentiation. Wiley Interdiscip Rev Syst Biol Med 2010;2:640-53. [Crossref] [PubMed]
- Mahla RS. Stem cells applications in regenerative medicine and disease therapeutics. Int J Cell Biol 2016;2016.
- Krause DS, Theise ND, Collector MI, et al. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell 2001;105:369-77. [Crossref] [PubMed]
- Clarke DL, Johansson CB, Wilbertz J, et al. Generalized potential of adult neural stem cells. Science 2000;288:1660-3. [Crossref] [PubMed]
- Mimeault M, Hauke R, Batra SK. Stem cells: a revolution in therapeutics-recent advances in stem cell biology and their therapeutic applications in regenerative medicine and cancer therapies. Clin Pharmacol Ther 2007;82:252-64. [Crossref] [PubMed]
- Yang ZF, Ho DW, Ng MN, et al. Significance of CD90+ cancer stem cells in human liver cancer. Cancer Cell 2008;13:153-66. [Crossref] [PubMed]
- Soltysova A, Altanerova V, Altaner C. Cancer stem cells. Neoplasma 2005;52:435. [PubMed]
- Li C, Heidt DG, Dalerba P, et al. Identification of pancreatic cancer stem cells. Cancer Res 2007;67:1030-7. [Crossref] [PubMed]
- Fathi E, Farahzadi R. Enhancement of osteogenic differentiation of rat adipose tissue-derived mesenchymal stem cells by zinc sulphate under electromagnetic field via the PKA, ERK1/2 and Wnt/beta-catenin signaling pathways. PLoS One 2017;12:e0173877. [Crossref] [PubMed]
- Sell S. Stem cells handbook. Springer, 2013.
- Sherman AB, Gilger BC, Berglund AK, et al. Effect of bone marrow-derived mesenchymal stem cells and stem cell supernatant on equine corneal wound healing in vitro. Stem Cell Res Ther 2017;8:120. [Crossref] [PubMed]
- Lindenmair A, Hatlapatka T, Kollwig G, et al. Mesenchymal stem or stromal cells from amnion and umbilical cord tissue and their potential for clinical applications. Cells 2012;1:1061-88. [Crossref] [PubMed]
- Gholizadeh-Ghalehaziz S, Farahzadi R, Fathi E, et al. A mini overview of isolation, characterization and application of amniotic fluid stem cells. Int J Stem Cells 2015;8:115. [Crossref] [PubMed]
- Mobarak H, Fathi E, Farahzadi R, et al. L-carnitine significantly decreased aging of rat adipose tissue-derived mesenchymal stem cells. Vet Res Commun 2017;41:41-7. [Crossref] [PubMed]
- Farahzadi R, Fathi E, Mesbah-Namin SA, et al. Zinc sulfate contributes to promote telomere length extension via increasing telomerase gene expression, telomerase activity and change in the TERT gene promoter CpG island methylation status of human adipose-derived mesenchymal stem cells. PLoS One 2017;12:e0188052. [Crossref] [PubMed]
- Fathi E, Farahzadi R. Zinc Sulphate Mediates the Stimulation of Cell Proliferation of Rat Adipose Tissue-Derived Mesenchymal Stem Cells Under High Intensity of EMF Exposure. Biol Trace Elem Res 2018;184:529-35. [Crossref] [PubMed]
- Mason C, Dunnill P. A brief definition of regenerative medicine. Regen Med 2008;3:1-5. [Crossref] [PubMed]
- Lai CY, Wu PJ, Roffler SR, et al. Clearance kinetics of biomaterials affects stem cell retention and therapeutic efficacy. Biomacromolecules 2014;15:564-73. [Crossref] [PubMed]
- Eckert MA, Vu Q, Xie K, et al. Evidence for high translational potential of mesenchymal stromal cell therapy to improve recovery from ischemic stroke. J Cereb Blood Flow Metab 2013;33:1322-34. [Crossref] [PubMed]
- Davies B, Elwood NJ, Li S, et al. Human cord blood stem cells enhance neonatal right ventricular function in an ovine model of right ventricular training. Ann Thorac Surg 2010;89:585-93.e4. [Crossref] [PubMed]
- Nagaya N, Kangawa K, Itoh T, et al. Transplantation of mesenchymal stem cells improves cardiac function in a rat model of dilated cardiomyopathy. Circulation 2005;112:1128-35. [Crossref] [PubMed]
- Roura S, Pujal JM, Gálvez-Montón C, et al. Impact of umbilical cord blood-derived mesenchymal stem cells on cardiovascular research. Biomed Res Int 2015;2015:975302. [Crossref] [PubMed]
- Ammar HI, Sequiera GL, Nashed MB, et al. Comparison of adipose tissue-and bone marrow-derived mesenchymal stem cells for alleviating doxorubicin-induced cardiac dysfunction in diabetic rats. Stem Cell Res Ther 2015;6:148. [Crossref] [PubMed]
- Camacho P, Fan H, Liu Z, et al. Small mammalian animal models of heart disease. Am J Cardiovasc Dis 2016;6:70. [PubMed]
- Camacho P, Fan H, Liu Z, et al. Large Mammalian Animal Models of Heart Disease. J Cardiovasc Dev Dis 2016;3:30. [Crossref] [PubMed]
- Hosseini Mansoub N, Gurdal M, Karadadas E, et al. The role of PRP and adipose tissue-derived keratinocytes on burn wound healing in diabetic rats. Bioimpacts 2018;8:5-12. [Crossref] [PubMed]
- Srionrod N, Bootcha R, Petchdee S. Foal Deciduous Teeth Stem Cells Enhance Wound Healing in Rabbit Wound Model. The Thai Journal of Veterinary Medicine 2016;46:155-61.
- Amirkhani MA, Shoae-Hassani A, Soleimani M, et al. Rejuvenation of facial skin and improvement in the dermal architecture by transplantation of autologous stromal vascular fraction: a clinical study. Bioimpacts 2016;6:149-54. [Crossref] [PubMed]
- Lin Y-C, Grahovac T, Oh SJ, et al. Evaluation of a multi-layer adipose-derived stem cell sheet in a full-thickness wound healing model. Acta Biomaterialia 2013;9:5243-50. [Crossref] [PubMed]
- Khorsand A, Eslaminejad MB, Arabsolghar M, et al. Autologous dental pulp stem cells in regeneration of defect created in canine periodontal tissue. J Oral Implantol 2013;39:433-43. [Crossref] [PubMed]
- Khazaei M, Bozorgi A, Khazaei S, et al. Stem cells in dentistry, sources, and applications. Dental Hypotheses 2016;7:42. [Crossref]
- Liu SP, Fu RH, Huang SJ, et al. Stem cell applications in regenerative medicine for neurological disorders. Cell Transplant 2013;22:631-7. [Crossref] [PubMed]
- Lee JK, Jin HK, Bae JS. Bone marrow-derived mesenchymal stem cells reduce brain amyloid-β deposition and accelerate the activation of microglia in an acutely induced Alzheimer's disease mouse model. Neurosci Lett 2009;450:136-41. [Crossref] [PubMed]
- Liu Z, Wang C, Wang X, et al. Therapeutic effects of transplantation of as-mir-937-expressing mesenchymal stem cells in murine model of alzheimer's disease. Cell Physiol Biochem 2015;37:321-30. [Crossref] [PubMed]
- Åkerud P, Canals JM, Snyder EY, et al. Neuroprotection through delivery of glial cell line-derived neurotrophic factor by neural stem cells in a mouse model of Parkinson's disease. J Neurosci 2001;21:8108-18. [Crossref] [PubMed]
- Venkataramana NK, Kumar SK, Balaraju S, et al. Open-labeled study of unilateral autologous bone-marrow-derived mesenchymal stem cell transplantation in Parkinson's disease. Transl Res 2010;155:62-70. [Crossref] [PubMed]
Cite this article as: Rajabzadeh N, Fathi E, Farahzadi R. Stem cell-based regenerative medicine. Stem Cell Investig 2019;6:19.




