
Investigation of multicellular tumor spheroids enriched for a cancer stem cell phenotype
Introduction
In recent years, there is a lot of data about the presence of cells at various stages of differentiation and proliferative potential in tumors (1). Some of these cells may be SCs (SC), that gives rise to new tumor clones (2). Normal SCs play an important role in maintaining homeostasis of tissues and organs. Due to the fact that normal SCs are slowly divided and have a longer life span than differentiated cells, they can be subjected to a variety of damaging factors and accumulate mutations that cause neoplasia (3). SC and cancer cells have some common properties, such as the ability to self-renewal and migration, the presence of telomeric and antiapoptotic mechanisms, increased activity of membrane transporters and the unity of ways to regulate self-renewal (4). Therefore, a small population of cells in a malignant tumor, characterized by an asymmetric division, the ability to self-renewal, replenishment of a pool of cancer cells and the promotion of the development of neoplasia are called cancer SCs (CSCs). Their number is less than 1% of all cells of the neoplasm, but they can significantly affect the development of the disease. There is evidence of the involvement of CSCs in the development of leukemia, myeloma, brain tumors, breast cancer (5). Some authors confirm that carcinogenesis in the mammary glands and other organs can lead to the transformation of resident SC and/or progenitor cells, which was caused by a violation of the regulation of the ways of self-renewal (6). Signal pathways in the body that regulate the self-renewal of SC, intensively interact with each other. Hedgehog and Notch signal pathways together form an inverse loop that regulates the normal development of the SC. The signals from both systems independently of each other affect the self-renewal of the SC, causing an increase in the expression of Bmi-1, which, along with the Wnt way, consider regulators of self-renewal of the SC (7). Only small subpopulations of CD133-positive cells of various human tumors behave like tumor-initiating cells (8). These cells proliferate in the cell culture in a non-differentiated state, support the growth of tumors of NOD/SCID mice after xenotransplantation and cause the formation of tumors that are not phenotypically different from primary human tumors. However, CSCs differ from tumor cells with the ability to self-regulate for a long time, the possibility of differentiation in progenitor cells (but not tumor cells) and high resistance to chemo and radiotherapy. Therefore, it is important to evaluate the effect of antitumor drugs on the population of the CSCs, since it plays a key role in tumor development (9).
The need for studying CSCs has stimulated the development of methods that allow specifically analyzing and isolating these cells. Detection of specific markers of CSCs, such as CD44, CD133, Oct4, Sox2, Nanog, ALDH-1, ABCG, CXCL12 and bmi-1 (10), promoted the active use of magnetic separators and cell sorters (11) to isolate these cells. At the same time, the method of cultivating multicellular tumor spheroids (MCTS) in vitro was developed (12). Tumor-derived spheroids (floating spheres) act as surrogate systems to evaluate the characteristics of CSCs in vitro. The main feature of such spheroids is the enrichment of the tumor population cells that have characteristics of the SC. Spheroid cell cultures are useful for modeling tissue architecture, studying the characteristics of signaling and microenvironment, invasion and immune response of cells during cancer development, as well as for studying the basic properties of CSCs (13).
Today, these cultures have an important application value, since they can serve as a new tool for early testing of drugs and potential therapies for the treatment of diseases (14). The use of spheroid cell cultures allows increasing the predictability of the efficacy and toxicity of the drugs, relative to the tumor population, before the drugs goes to clinical trials (15). The mechanisms of gene expression in spheroid cell cultures are closer to in vivo conditions compared to monolayer cell cultures (16). The possibility of enriching of the tumor population (in this case, MCTS) by the CSCs allows the in vitro studies that are close to the body conditions in the event of micrometastasis (17). Different sources and types of cancer cells can be used for the formation of tumor spheroids. General procedures for enriching MCTS by CSCs based on the unique ability of SC to survive and grow in the form of spherical structures in serum-free conditions with the addition of growth factors (18). The ability to form non-adhesive spheroids is demonstrated for CSCs of different origin (19). Compared to conventional monoclonal culture, MCTS supports the key properties of SC, including receptor profile, gene expression profiles, colony forming and oncogenic activity, high potential for differentiation, secretory activity and chemotherapy resistance (20,21). So, the standardization of cultivation protocols of MCTS enriched with CSCs (eMCTS) may provide the opportunity to use these cultures for the identification of drugs that can suppress the proliferation of CSCs.
The aim of this study was to investigate cell populations with the characteristics of SC and their sensibility to antitumor drugs (cysplatin, methotrexate and doxorubicin) on the model of MCTS enriched with CSCs (eMCTS).
Methods
2D cell culture
MCF-7 cell line (mammary gland adenocarcinoma) was kindly provided by the Bank of human and animal cell lines of the Institute of Experimental Pathology, Oncology and Radiobiology of the R.E. Kavetsky Institute of the National Academy of Sciences of Ukraine. Cells of this line were cultured under standard conditions (37 °C, 5% CO2, humidity 95%), in complete nutrient medium (CNM): DMEM (Sigma, USA), with 10% fetal bovine serum (FBS, Sigma, USA), 2 mM L-glutamine (Sigma, USA), 40 mg/mL gentamicin (Biopharma, Ukraine). The initial cell density was 2×104 cells/cm2. Cells were used in an experiment after two days of incubation.
3D cell culture (MCTS)
For the initial generation of MCTS, monolayer cell cultures (5×105 cells/mL) were removed from the substrate using 0.25% trypsin-EDTA and transferred to CNM containing an additional 2% carboxymethylcellulose (Bio-Rad, USA). Cells were incubated on PSU-10i orbital shaker (Biosan, Latvia) at 80 rpm for 3–5 hours. Half of the culture medium was changed every 3 days. The spheroid culture was maintained for 7 days.
3D spheroid cell culture enriched with CSCs (eMCTS)
The general scheme of eMCTS cultivation included the same manipulations as for conventional MCTS. However, these spheroids were cultured in serum-free conditions with the addition of a fibroblast growth factor (FGF, Sigma, USA), the epidermal growth factor (EGF, Sigma, USA, 20 µg/mL), insulin (5 µg/mL, farmasuline, Farmak, Ukraine) and hydrocortisone (1 µg/mL, hydrocortisone acetate, Farmak, Ukraine).
Evaluation of cell sensitivity to anticancer drugs
Antitumor drugs were added to 2D and 3D cultures in the concentrations: cisplatin 0.01, 0.1, 1 µg/mL; methotrexate 0.1, 1, 10 µg/mL; doxorubicin 0.1, 1, 10 µg/mL and incubated for 48 hours. Their effect on the tumor cells viability was investigated using the MTT test: 4 hours before the end of the incubation period 20 µL of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) solution (5 mg/ml phosphate-buffered buffer) was added to 100 µL of the cell suspension and continued incubation during 3 hours. After centrifugation (1,500 rpm, 5 min), a supernatant was removed. To dissolve the formazan crystals, 100 µL of dimethyl sulfoxide (DMSO, Serva, Ukraine) were added to each samplel. Optical absorption was measured using a Multiskan MCC/340 spectrophotometer at 540 nm. The obtained data were compared with optical absorption in control samples and calculated the percentage of viable cells comparing to control.
Calculation of live and dead cells
After the trypsinization of cells during recultivation of cell culture, it is possible to evaluate the state of the cell culture and to calculate the total number of living and dead cells. For these purposes, there is method for counting cells in a hemocytometer (Goryaev’s cell). An equal volume of 0.1% trypan blue is added to the cell suspension. This dye paints only dead cells.
Measurement of spheroids size
To analyze the effect of antitumor drugs on the volumes of MCTS and eMCTS in 3D culture, they were evaluated by photographing and measuring aggregate sizes after 2 days of incubation. The software Stemi2000 (Zeiss, Germany) was used for image processing and the volume of aggregates was calculated using the Bierkvig formula: V =0.4× a × b2, where a = greater diameter, b = smaller diameter, 0.4= coefficient, determined for spheroids (17).
Immunohistochemistry
To evaluate the expression of CSCs markers characteristic, 2D culture was recultivated on cover glass in 6-well plates at 2×104 cells/cm2. The MCTS and eMCTS were embedded into the histological blocks and the standard procedure for the preparation of permanent drugs was performed. Further immunohistochemical staining was performed according to the protocol recommended for the PolyVue© peroxidase detection system. Expression of the receptors was analyzed using primary monoclonal antibodies CD24 (Sigma, USA), CD44 (Sigma, USA), CD133 (Sigma, USA), bmi-1 (Sigma, USA), vimentin (Sigma, USA). The samples were colored with hematoxylin and fixed by Canadian balsam. Histological cell samples were photographed to compare the expression of receptors in both monolayer and spheroid cell cultures.
Statistical analysis
The one-factor analysis of dispersion and t-Student testing with the software package Statistica 8 was used for statistical data processing. The threshold was *P≤0.05. The results are presented as means and standard errors (M ± SE).
Results
Morphological analysis of CSCs population in eMCTS
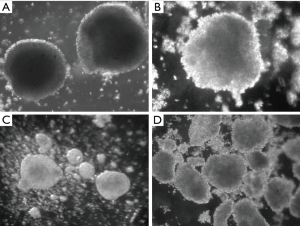
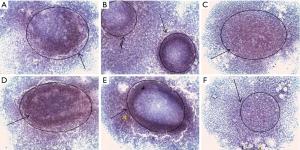
MCF-7 cells can form spheroids in suspension conditions in serum-free nutrient medium with addition of growth factors (Figure 1). On the 7th day of cultivation, cells of MCTS culture began to differ in morphology from eMCTS. They had a loose structure and a less pronounced necrotic core. The nutrient medium, in which these spheroids cultivated, contained more single floating cells, possibly due to lack of serum. Suspended cultivation in unsteady conditions, with the addition of growth factors necessary for the viability and division of SCs, creates a peculiar selection of cells. That is, tumor cells do not receive enough nutrients and some of them die and SCs experience unfavorable conditions and their development is supported by growth factors.
Receptor status study of CSCs populations in eMCTS
The next step in the study was to analize of expression of CSCs markers in monolayer and spheroidal growth. In this study, the ability of cell lines to include populations of cells that have similar characteristics with SCs in primary tumors has been shown. Therefore, one of the tasks was to evaluate the expression of CSC markers during cultivation in adhesive and suspension growth conditions, as well as in terms of cell enrichment with CSCs. It was found that adhesive cells of monolayer culture weakly (<10%) express markers of CD133, CD44, CD24 and bmi-1 (Figures 2,3). These markers expressed by individual cells, mainly on the periphery of the cellular layers, as well as cells that have lost the ability to contact inhibition and begin to form a second layer of cells, that is, “fill” each other in the center of the cell aggregate. Since cells that are positive for CD133, CD44, CD24 and bmi-1, have the ability to migrate and possess the characteristics of SCs, most likely these cells will initiate more aggressive cell clones and replenish a tumor cell pool in a 2D culture.
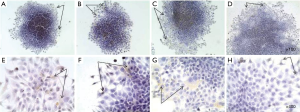
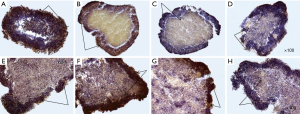
After transferring the adhesive cells to substrate-independent cell growth conditions, multicellular spheroids begin to express more (>10%) cells that are positive for CD133, CD44, CD24 and bmi-1 markers (Figure 3), with CD24 expression having less pronounced character, in comparison with other markers. The most pronounced expression of markers of CSCs is the cells of the outer layers of the spheroid. However, the inner layers of the spheroid also contain CSCs-positive cells, which, on its part, characterize a population of cells that can survive adverse conditions, because within the spheroid the cells receive less amount of nutrients and oxygen. As described above, for CSCs, hypoxic conditions are not critical, they may be in a dormant state under such conditions and are capable of self-renewal. Figure 3 shows the expression of the marker of self-renewal of bmi-1 both in the outer and in the inner layers of the spheroid. Expression of CD44 is more pronounced, compared to CD24. High expression of CD44 and low expression of CD24 are characteristic of CSCs of breast cancer. Immunohistochemical analysis showed that MCTS cultivated under normal suspension conditions (without the addition of any stimulatory factors) themselves have cell populations with stem characteristics. However, it is possible to increase the percentage of such cells in the MCTS by cultivating in the serum-free conditions and by adding growth factors (growth factor of fibroblasts, epidermal growth factor) that promote the growth of SCs and limiting the active growth of tumor cells.
After enriching the suspension culture with cells with characteristics of CSCs (eMCTS), the intensity of expression of surface markers of CSCs was also analyzed. Of course, such analysis is only qualitative, since it is difficult to assess the quantitative characteristics of the markers in terms of the immunogenic chemistry method, but it is planned to further explore the percentage of CSCs using the cytofluorimetry method in the future. At this stage, it has been shown that the cells of the eMCTS surface layers have overexpression (>20%) of the markers bmi-1, CD44 and CD133, however, the expression of CD24 is less pronounced, as in conventional MCTS and monolayer cell culture. Thus, using the immunohistochemistry method, it has been shown that eMCTS contains the population of cells with the most pronounced expression of CSCs markers, as compared to the adhesion culture and tumor spheroids cultivated without growth factors. Detection of high-level CSCs markers in eMCTS may be useful for CSCs research and for testing of anti-tumor therapies directed at these cells.
Comparison of the sensitivity of 2D cell culture and MCTS to the antitumor drugs effect
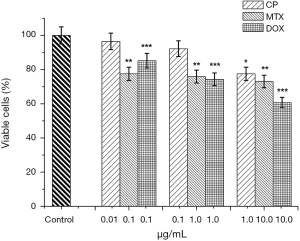
The following drugs were selected for evaluation of the effect of antitumor therapy on tumor cells of the mammary gland: cisplatin (CP), doxorubicin (DOX) and methotrexate (MTX). When MCF-7 cells were incubated in a monolayer culture with 0.01 µg/mL cisplatin, cell viability was 96.5%; at 0.1 µg/mL, 92.2% and 1.0 µg/mL, 77.5%, relative to control (Figure 4). As a result of incubation MCF-7 cells of 2D cell culture with 0.1, 1.0 and 10.0 µg/mL MTX (Figure 4) viability of tumor cells was 77.5%, 75.9% and 73.0% of the control, respectively.
When incubating MCF-7 cells in the spheroid culture, an increase in CP concentration resulted in a 10% (0.01 µg/mL), 50% (0.1 µg/mL) and 90% (1.0 µg/mL) decrease in MCTS volume relative to control (Figure 5A). The average volume of MCTS with an increase in cisplatin concentration is also reduced by 40% at 0.01 µg/mL, 60% at 0.1 µg/mL and 66% at 1.0 µg/mL.
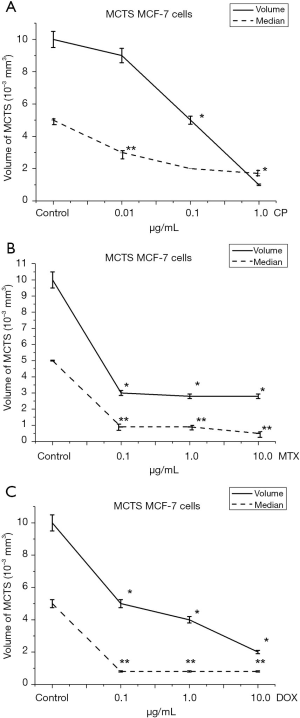
At the same time, when incubating a spheroid culture in the presence of MTX, at the same concentrations, the volume of spheroids decreased by 50%, 60%, 80% (Figure 5B) relative to control. It is noteworthy that the median volume of MCTS decreased at the lowest concentration (0.1 µg/mL), by 84% and remained at 0.8×10−3 mm3 with a further increase in MTX concentration. An explanation of MCF-7 cell resistance can be found in the mechanism of action of MTX. MTX is an effective anti-tumor agent with a wide range of clinical applications. MTX strongly inhibits the DHF-reductase enzymes (22). Previous studies have identified three possible mechanisms for resistance to MTX (MTX-R): (I) the presence of defects in drug delivery to the cell, (II) changes in DHF-reductase activity, resulting from decreased affinity for MTX and (III) increased levels of DHF-reductase. Since the rate of drug transport to MCF-7 cells in the spheroid culture decreases, the level of free intracellular MTX, which is available for subsequent conversion to polyglutamates in the spheroid culture, is lower than that of monolayer cells. However, even the incubation of 3D cells in conditions whereby the level of free intercellular MTX increases, did not cause dose-dependent MCF-7 cell sensitivity. Thus, with the incubation of the 3D cells of MCF-7 with increasing of MTX concentration from, 0.1 to 10.0 µg/mL, cell sensitivity was not statistically significant.
We assume a high level of DHF-reductase activity in the used cell line, which led to reduced cell viability at low concentrations with subsequent exhaustion of the substrate and the maintenance of the percentage of living cells at 70.0% in the monolayer culture and the maintenance of the average volume MCTS at the level of 2.8×10−3 mm3. Incubation of MCF-7 cells in monolayer culture demonstrated dose-dependent sensitivity to Dox (Figure 4). Minimum concentration of Dox was 0.1 µg/mL, the percentage of alive cells was decreased to 85.2%. With further increase in Dox concentration to 1.0 and 10.0 µg/mL, the viability of tumor cells decreased to 74.3% and 60.7% relative to control. When MCF-7 cells were incubated in a spheroid culture (Figure 5C), a dose-dependent decrease in the volume of MCTS was observed. In the control samples the average volume of MCTS was 10.0×10−3 mm3. With 0.1 µg/mL Dox, the volume of MCTS was 5.0×10−3 mm3. With further increase of Dox concentration to 1.0 and 10.0 µg/mL, the volume of MCTS was 4.0×10−3 mm3 and 2.0×10−3 mm3, respectively.
Figures 4,5 show that MCF-7 cells have different sensitivities to drugs of different mechanisms of action. Monolayer and spheroidal cultures showed the same tendency for correlation of concentrations of investigated substances and viability of tumor populations. It was found that Dox has the greatest cytotoxic effect on MCF-7 cells in both monolayer and spheroid cultures. Instead, methotrexate had a significant cytotoxic effect at low concentrations (0.1 µg/mL), but a further increase of concentration did not lead to a decrease in cell viability in both monolayer and spheroid cultures. Similarly, the dependence of the viability of tumor cells on the concentration of the active substance was repeated in monolayer and spheroid cultures when incubated with CP. Thus, when comparing the viability of MCF-7 cells in monolayer culture and the growth of MCF-7 multicellular spheroidal tumors under the influence of CP, Dox and MTX, identical trends of sensitivity and resistance were obtained.
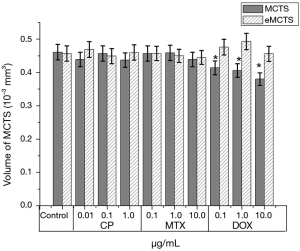
Comparing the average volumes of MCTS and eMCTS shows that eMCTS culture is less sensitive to anti-tumor drugs on the second day of cultivation (Figure 6). Thus, the cytotoxic effect of Dox was shown in MCTS with the spheroid volume reduced by 12% and 19% with 1 µg/mL and 10 µg/mL Dox, respectively, compared with control spheroids. However, Dox did not significantly reduce the average eMCTS volume. CP and MTX practically do not affect the volume of cellular aggregates. Dox leads to a slight change in the volumes of cellular aggregates: in the case of CP and MTX, they decrease; in the case of Dox, they even slightly increase.
Comparison sensitivity of MCF-7 cells to anticancer drugs in 2D culture, MCTS and eMCTS
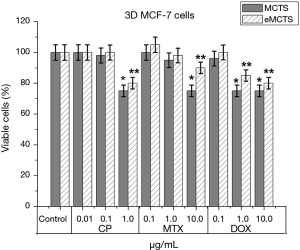
Comparing tumor cell sensitivity to anti-tumor drugs in 2D culture (Figure 4), MCTS and eMCTS (Figure 7), show that all three cultures are equally sensitive to CP activity. Increasing of CP concentration from 0.01 to 0.1 µg/mL practically does not affect cell viability in MCTS and eMCTS cell cultures, but in the 2D culture, the cell viability decreased by 8%. With 1.0 µg/mL CP, viability of 2D, MCTS and eMCTS cultures decreases to 77.5%, 75% and 80%, respectively, as compared to control. 2D cell culture was the most sensitive to MTX, with viability of cells decreasing almost identically for all concentrations studied. For MCTS, the culture with 0.1 and 1.0 µg/mL MTX practically did not affect cell viability, and with 10.0 µg/mL MTX viability was reduced to 76%. In MCTS, the viability of cells with an increase in the concentration of MTX only slightly decreased, and at a maximum concentration of MTX was 90%. The highest sensitivity to doxorubicin was observed in 2D cultures, somewhat lower in MCTS and the smallest in eMCTS. Increasing the concentration of Dox leads to a sharp decrease in viability in 2D culture (up to 60.7%) and to a marked decrease in MCTS and eMCTS culture (at maximum concentrations 75% and 80%, respectively).
Reversion of eMCTS from suspension to adhesive growth
Following the generation of eMCTS by anchorage-independence, we next induced the reversal of spheroids by transferring them into adhesive culture condition. During the reversal process, cells were observed to migrate in a “down-hill” fashion from the vertical top axis of the spheroid to its base which is close to the attachment/adhesive-surface, but in a direction away from the spheroid-base.
After that, eMCTS was attached to the surface of the culture plate, and cells of its surface layers began to proliferate. Special attention in our study was focused on the expression of CSCs markers in such conditions. It was found (Figure 8) that the cells of the eMCTS surface layers were enriched with markers of CSCs (CD133, CD44, CD24, bmi-1, EpCAM, vim). They give rise to tumor cells themselves, for which the expression of the corresponding markers is not characteristic. Results of the current study demonstrate that reversed-spheroid population possesses aggressive phenotypic characteristics such as invasion, cancer stem cell markers and chemoresistance.
Discussion
It is known that CSCs are the most radio- and chemo-resistant populations in the tumor of a wide range of malignant neoplasms, including breast cancer. It is important that the experimental model of the disease reproduces in vivo conditions as much as possible. The cellular model for breast cancer research must be adequate in terms of target receptor expression, enzyme activity and the interaction of individual cells and extracellular environment. For many years, cell cultures have been widely used for fundamental research and are an integral part of antitumor drug testing, but most of them still hold in monolayer (2D) growth. Such cultures, of course, are easily maintained and controlled in laboratory conditions. However, in the body there are no cells that live exclusively in two-dimensional space. Cells interact constantly with each other, forming intercellular contacts, exchanging substances and diverse signals. The extracellular matrix and microenvironment play a decisive role. The advantages of 3D cultivation are the preservation of the polarity and cell morphology, as well as the nature of gene expression and activation of intracellular cascades characteristic of the original tissue (23). 3D cell culture (MCTS) is also an important way of enriching the culture by CSCs in vitro and deserves special attention during fundamental research and drug screening.
The eMCTS method of cultivation is a way of maintaining the CSCs population in culture in close cooperation with cancer cells. The CSCs population in the substrate-independent growth is expected to maintain its ability to self-renew, expressing of specific markers and support the growth of the tumor cell population. The authors believe that eMCTS heterogeneous model, consisting of both cancer and tumor cells, has advantages over cultures isolated by CSCs cell separators. Cells have the potential for mutual influence by forming intercellular contacts and exchanging cytokines and growth factors. The main concern of CSCs markers is their nonspecificity, because CSCs express different markers and their combinations in different tumors. In vivo these markers can be detected not only on CSCs, but also on resident SCs or precursor cells, on tumor stromal elements or tumor cells without stem properties. Therefore, only a part of the cells with this marker may be CSCs (24). However, there is a combination of CSCs markers that are specific to breast cancer (CD133, CD44, CD24, ALDH, etc.). Methods of magnetic separation and cell sorting have shown that the isolated population of breast cells with high CD44 and with low CD24 expression has all the properties characteristic of CSCs, therefore, such a combination of markers is used to detect the population of these cells in breast tumors (25,26). CD44 is a transmembrane glycoprotein that plays an important role in cell division, migration, adhesion and signaling (27). It is usually expressed in both embryonic and adult hematopoietic SCs. High expression of CD44 is characteristic for many types of cancer, particularly for breast cancer (28). Another important marker of CSCs is CD133, which is a transmembrane glycoprotein and is expressed by hematopoietic SC, endothelial cells from precursors (29), in glioblastoma, neuronal and glial SC (30) and also involved in cell growth and development (31). Tumor cells with the CD133 + phenotype exhibit specific SC characteristics such as self-renewal, differentiation and tumor formation on NOD-SCID mice models. After injected CD133 + cells into immunodeficient mice, they exhibit chemo and radio resistance (32). There are also proteins associated with self-updating CSCs. Thus, bmi-1 is a protein necessary for the self-healing of hematopoiesis and nerve SC (33). Induced expression of bmi-1 drugs increases the population of CSCs on head and neck cancer models (34) and is a marker of CSCs (35) and a poor prognosis during breast cancer (36). Bmi-1 plays an important role in the proliferation, invasion and metastasis of cancer cells and resistance to anti-cancer therapy, as well as in maintaining the properties of CSCs (37).
Cisplatin (CP), doxorubicin (DOX) and methotrexate (MTX) were selected for evaluation of the effect of antitumor therapy on tumor cells. CP binds to the bases of DNA and inhibits DNA synthesis as a result of cross-linking within the DNA strands and between them. Synthesis of protein and RNA are lesser suppressed. Although the antitumor effect of cisplatin is mainly due to inhibition of DNA synthesis, there are other mechanisms of its anti-neoplastic action. In particular, cisplatin increases the immunogenicity of tumors. CP also has immunosuppressive and antibacterial properties and increases cell sensibility to irradiation. The effect of cisplatin on cells does not depend on the phase of the cycle. As a platinum-based drug, cisplatin (38) implements the cytotoxic effect through several mechanisms. One of the mechanisms is DNA interaction with the formation of G-G DNA-transverse bonds, which leads to the activation of several ways of transmitting the DNA damage signal and the induction of mitochondrial apoptosis (39). However, there is evidence of the development of chemoresistance and therapeutic insufficiency of cisplatin (40). Therefore, circuits for combining CP with chemosensibilizers or synergists, which are potentially able to improve the efficacy of treatment and restore sensitivity to cisplatin, have been developed. MTX, as one of the chemotherapeutic agents, is commonly used to treat metastatic breast cancer, acute myeloid leukemia and non-Hodgkin's lymphoma (41). MTX prevents proliferation of cells by inhibiting the activity of topoisomerase II and DNA repair/synthesis (42), intercalation to DNA (43), DNA damage and induction of apoptosis by inhibition of the mitochondrial pathway (44). Unfortunately, MTX has a non-specific effect on healthy cells (45). In addition, cancer cells can become resistant to MTX. According to the literature, possible explanations for MCF-7 cell resistance to MTX may be either reduction of DHF-reductase activity and low MTX transformation or a significant increase in the activity of the enzyme preparation in the cells. Both ways lead to a huge increase in the excess of free levels of MTX in 24 hours. Thus, a decrease in the concentration of free MTX in these cells, with high DHF-reductase activity, leads to a restriction of the free substrate and a decrease in the total number of formed MTX polyglutamates. According to literature (46), the MCTS culture is less sensitive to the action of antitumor drugs, since the main mechanism of chemo-resistant CSCs is the rapid removal of these anti-tumor drugs from these cells due to the high expression of ATP-binding cassette proteins on the cell membrane carrying out reverse toxicity of the extracellular space. It has been shown that inhibition of such transporters leads to an increase in the sensitivity of CSCs to the action of antitumor drugs. In addition, CSCs contain a large amount of aldehyde dehydrogenase (ALDH1), an enzyme that inactivates most chemotherapeutic drugs. It is also known that CSCs differ in their extreme resistance to apoptosis. In cases then resistance systems fail, CSCs go to rest (dormancy), which may last for decades and then activate and cause a fatal recurrence. Dormant CSCs have very low levels of metabolism, extreme resistance to damaging effects and almost no surface protein expression that could be used for targeted therapy (47). Since the presence of CSCs has a significant effect on the sensitivity of MCTS to anticancer therapy, the authors compared the effect of anticancer drugs on the culture of MCTS and eMCTS, which contain a larger number of cells with stem characteristics.
In general, eMCTS culture exhibits the least sensitivity to investigated drugs. In was shown that the stem cell phenotype of breast cancer cells in eMCTS was further verified by the high resistance of the cells to chemotherapy. CSCs were shown to have high resistance to therapy, probably due to their slow cell cycle and they are more resistant to apoptosis than differentiated cells. CSCs have high expression levels of the Bcl-2 family genes, ABC transporters such as BCRP and multidrug resistance associated proteins, all of which are known to play important roles in drug resistance. Moreover, the culture of MCTS is less sensitive to antitumor therapy due to limited diffusion of drugs to the inner layers of the spheroid. The high sensitivity of 2D culture to the investigational drugs indicates that the 2D model is ineffective in the study of anticancer drugs. Therefore, testing antitumor drugs on a multicellular tumor spheroid-rich multicellular model is an important step in preclinical testing, which allows for more complete information on the drug's potential to affect heterogeneous tumor the population. One of the benefits of MCTS, which grows under substrate-independent growth, is the selection of cells with a clonogenic or aggressive phenotype. The MCTS simulates the characteristic properties of metastases, such as migration, invasion, chemoresistance and the presence of stem-like cells. Similar experiment with reversion eMCTS from suspension to adhesive growth was conducted by Kunjithapatham and colleagues (48). They showed that a spheroid transmitted from a non-adhesive to an adhesive culture had an aggressive phenotype, its cells express CSC markers and have a low sensitivity to the drugs. In addition, the expression of vimentin, a mesenchymal marker with an epithelial-mesenchymal transition (EMT), is associated with the migratory ability of tumor cells and poor prognosis during breast cancer. Increasing its level in spheroid can also indicate the initial stages of EMT, which is a critical determinant of the spread of tumor cells. Thus, the approach of authors to the development of MCTS and eMCTS is based exclusively on cell cultivation in non-adherent growth conditions without auxiliary materials that could influence the development of the tumor process, including EMT.
Results of the current study demonstrate that eMCTS population possesses aggressive phenotypic characteristics such as invasion, expression of cancer stem cell markers and chemoresistance. The increased expression of CD133, CD44, CD24 and bmi-1 markers in eMCTS was detected in comparison with the adhesive cell culture and MCTS cultured under standard conditions. The eMCTS culture is less sensitivity to the antitumor drugs (cisplatin, methotrexate and doxorubicin) compared to the MCTS cultured under standard conditions. We demonstrate that the malignant characteristics of eMCTS are maintained even after the reversal of phenotype into a monolayer. In summary, eMCTS incorporates clonogenic capacity, migratory and invasive properties along with low sensitivity to anticancer drugs. It is suggest about all the metastatic characteristics to investigate its biology and regulation. eMCTS could be valuable in understanding the regulation of CSCs functions which in turn could provide insights into potential therapeutic targets.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Al-Hajj M, Wicha MS, Benito-Hernandez A, et al. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A 2003;100:3983-8. [Crossref] [PubMed]
- Ayob AZ, Ramasamy TS. Cancer stem cells as key drivers of tumor progression. J Biomed Sci 2018;25:20-4. [Crossref] [PubMed]
- Dontu G, Al-Hajj M, Abdallah WM, et al. Stem cells in normal breast development and breast cancer. Cell Prolif 2003;36:59-72. [Crossref] [PubMed]
- Madka V, Rao CV. Cancer stem cell markers as potential targets for epithelial cancers. Indian J Exp Biol 2011;49:826-35. [PubMed]
- Clevers H. The cancer stem cell: premises, promises and challenges. Nat Med 2011;17:313-9. [Crossref] [PubMed]
- Liu S, Dontu G, Wisha M. Mammary stem cells, self-renewal pathways and carcinogenesis. Breast Cancer Res 2005;7:86-95. [Crossref] [PubMed]
- Templeton AK, Miyamoto S, Babu A, et al. Cancer stem cells: progress and challenges in lung cancer. Stem Cell Investig 2014;1:9. [PubMed]
- Lou N, Wang Y, Sun D, et al. Isolation of stem-like cells from human MG-63 osteosarcoma cells using limiting dilution in combination with vincristine selection. Indian J Biochem Biophys 2010;47:340-7. [PubMed]
- Cohen AA, Geva-Zatorsky N, Eden E, et al. Dynamic proteomics of individual cancer cells in response to a drug. Science 2008;322:1511-6. [Crossref] [PubMed]
- Prud’homme GJ. Cancer stem cells and novel targets for antitumor strategies. Curr Pharm Des 2012;18:2838-49. [Crossref] [PubMed]
- Dobbin ZC, Landen CN. Isolation and Characterization of Potential Cancer Stem Cells from Solid Human Tumors – Potential Applications. Curr Protoc Pharmacol 2013;63:Unit 14.28.
- Weiswald LB, Bellet D, Dangles-Marie V. Spherical cancer models in tumor biology. Neoplasia 2015;17:1-15. [Crossref] [PubMed]
- Nath S, Devi GR. Three-dimensional culture systems in cancer research: Focus on tumor spheroid model. Pharmacol Ther 2016;163:94-108. [Crossref] [PubMed]
- Breslin S, O’Driscoll L. Three-Dimensional Cell Culture: The Missing Link in Drug Discovery. Drug Discov Today 2013;18:240-9. [Crossref] [PubMed]
- Fang Y, Eglen RM. Three-dimentional cell culture in drug discovery and development. SLAS Discovery 2017;22:456-72. [Crossref] [PubMed]
- Souza AG, Silva IB. Comparative Assay of 2D and 3D Cell Culture Models: Proliferation, Gene Expression and Anticancer Drug Response. Curr Pharm Des 2018;24:1689-94. [Crossref] [PubMed]
- Bjerkvig R. Spheroid culture in cancer research. CRC Press, Boca Raton, 1992.
- Pastrana E, Silva-Vargas V, Doetsch F. Eyes wide open: a critical review of sphere-formation as an assay for stem cells. Cell Stem Cell 2011;8:486-98. [Crossref] [PubMed]
- Tang C, Ang B, Pervaiz S. Cancer stem cell: target for anti-cancer therapy. FASEB J 2007;21:3777-85. [Crossref] [PubMed]
- Krohn A, Song YH, Muehlberg F, et al. CXCR4 receptor positive spheroid forming cells are responsible for tumor invasion in vitro. Cancer Lett 2009;280:65-71. [Crossref] [PubMed]
- Ishiguro T, Ohata H, Sato A, et al. Tumor derived spheroids: Relevance to cancer stem cells and clinical applications. Cancer Science 2017;108:283-9. [Crossref] [PubMed]
- Minotti G, Menna P, Salvatorelli E, et al. Anthracyclines: molecular advances and pharmacologic developmentsin antitumor activity and cardiotoxicity. Pharmacol Rev 2004;56:185-229. [Crossref] [PubMed]
- Yamada KM, Cukierman E. Modeling tissue morphogenesis and cancer in 3D. Cell 2007;130:601-10. [Crossref] [PubMed]
- Sneddon JB, Werb Z. Location, location, location: the cancer stem cell niche. Cell. Stem Cell 2007;1:607-11. [Crossref] [PubMed]
- Idowu MO, Kmieciak M, Dumur C, et al. CD44+/CD24−/low cancer stem/progenitor cells are more abundant in triple-negative invasive breast carcinoma phenotype and are associated with poor outcome. Hum Pathol 2012;43:364-73. [Crossref] [PubMed]
- Bauerschmitz GJ, Ranki T, Kangasniemi L, et al. Tissue-specific promoters active in CD44+CD24−/low breast cancer cells. Cancer Res 2008;68:5533-9. [Crossref] [PubMed]
- Basakran NS. CD44 as a potential diagnostic tumor marker. Saudi Med. J. 2015;36:273-9. [Crossref] [PubMed]
- Joshua B, Kaplan MJ, Doweck I, et al. Frequency of cells expressing CD44, a head and neck cancer stem cell marker: correlation with tumor aggressiveness. Head Neck 2012;34:42-9. [Crossref] [PubMed]
- Handgretinger R, Gordon PR, Leimig T, et al. Biology and plasticity of CD133+ hematopoietic stem cells. Ann N Y Acad Sci 2003;996:141-51. [Crossref] [PubMed]
- Singh SK, Clarke ID, Terasaki M, et al. Identification of a cancer stem cell in human brain tumors. Cancer Res 2003;63:5821-8. [PubMed]
- Li Z. CD133: a stem cell biomarker and beyond. Exp Hematol Oncol 2013;2:17-21. [Crossref] [PubMed]
- Singh SK, Hawkins C, Clarke ID, et al. Identification of human brain tumor initiating cells. Nature 2004;432:396-401. [Crossref] [PubMed]
- Molofsky AV, Pardal R, Iwashita T, et al. Bmi-1 dependence distinguishes neural stem cell self-renewal from progenitor proliferation. Nature 2003;425:962-7. [Crossref] [PubMed]
- Nör C, Zhang Z, Warner KA, et al. Cisplatin induces Bmi-1 and enhances the stem cell fraction in head and neck cancer. Neoplasia 2014;16:137-46. [Crossref] [PubMed]
- Liu S, Dontu G, Mantle ID, et al. Hedgehog signaling and Bmi-1 regulate self-renewal of normal and malignant human mammary stem cells. Cancer Res 2006;66:6063-71. [Crossref] [PubMed]
- Wang Y, Zhe H, Ding Z, et al. Cancer stem cell marker Bmi-1 expression is associated with basal-like phenotype and poor viability in breast cancer. World J Surg 2012;36:1189-94. [Crossref] [PubMed]
- Wang MC, Li CL, Cui J, et al. BMI-1, a promising therapeutic target for human cancer. Oncol Lett 2015;10:583-8. [Crossref] [PubMed]
- Galluzzi L, Senovilla L, Vitale I, et al. Molecular mechanisms of cisplatin resistance. Oncogene 2012;31:1869-83. [Crossref] [PubMed]
- Barar J, Kafil V, Majd MH, et al. Multifunctional mitoxatrone-conjugated magnetic nanosystem for targeted therapy of folate receptor-overexpressing malignant cells. J Nanobiotechnology 2015;13:26. [Crossref] [PubMed]
- Crespi MD, Ivanier SE, Genovese J, et al. Mitoxantrone affects topoisomerase activities in human breast cancer cells. Biochem Biophys Res Commun 1986;136:521-8. [Crossref] [PubMed]
- Mazerski J, Martelli S, Borowski E. The geometry of intercalation complex of antitumor mitoxantrone and ametantrone with DNA: molecular dynamics simulations. Acta Biochim Pol 1998;45:1-11. [PubMed]
- Ferrer A, Marce S, Bellosillo B, et al. Activation of mitochondrial apoptotic pathway in mantle cell lymphoma: high sensitivity to mitoxantrone in cases with functional DNA-damage response genes. Oncogene 2004;23:8941-9. [Crossref] [PubMed]
- Neidhart JA, Gochnour D, Roach R, et al. A comparison of mitoxantrone and doxorubicin in breast cancer. J Clin Oncol 1986;4:672-7. [Crossref] [PubMed]
- Avilés A, Arevila N, Diaz Maqueo JC, et al. Late cardiac toxicity of doxorubicin, epirubicin and mitoxantrone therapy for Hodgkin’s disease in adults. Leuk Lymphoma 1993;11:275-9. [Crossref] [PubMed]
- Aljarrah K, Mhaidat NM, Al-Akhras MA, et al. Magnetic nanoparticles sensitize MCF-7 breast cancer cells to doxorubicin-induced apoptosis. World J Surg Oncol 2012;10:62-9. [Crossref] [PubMed]
- Alison MR, Guppy NJ, Lim SML. Finding cancer stem cells: are aldehyde ehydrogenases fit for purpose? J Pathol 2010;222:335-44. [Crossref] [PubMed]
- Klein CA. Parallel progression of primary tumors and metastases. Nat Rev Cancer 2009;9:302-12. [Crossref] [PubMed]
- Kunjithapatham R, Karthikeyan S, Geschwind JF. Reversal of Anchorage-Independent Multicellular Spheroid into a Monolayer Mimics a Metastatic Model. Sci Rep 2014;4:6816-20. [Crossref] [PubMed]
Cite this article as: Herheliuk T, Perepelytsina O, Ugnivenko A, Ostapchenko L, Sydorenko M. Investigation of multicellular tumor spheroids enriched for a cancer stem cell phenotype. Stem Cell Investig 2019;6:21.


