
Spontaneous tumor lysis syndrome in T-cell malignancy: two case reports
Introduction
Tumor lysis syndrome (TLS) is a term used to describe a constellation of metabolic abnormalities (hyperuricemia, hypocalcemia, hyperphosphatemia, and hyperkalemia) that result from a rapid turn-over of malignant cells (1). In most cases, TLS is triggered by intensive chemotherapy of aggressive, bulky tumors that lead to massive cell death and consequent release of intracellular constituents—mainly potassium, phosphate, and products of nucleic acid metabolism. Uric acid—a by-product of nucleic acid metabolism—can precipitate inside renal tubules and form crystals in the urine, thereby leading to obstruction of urinary flow and resulting in acute kidney injury (2). Spontaneous TLS is a rarely described entity that can occur in patients with highly aggressive liquid or solid malignancies (3). TLS is a potentially fatal oncologic emergency and it is crucial that clinicians recognize the laboratory and clinical findings that suggest this condition. This diagnosis should be considered even in the absence of an established malignancy because spontaneous TLS may be the initial presentation of occult or untreated cancers. Given its high morbidity and mortality, it must be recognized early in order to start appropriate treatment. Here, we describe two patients who presented with spontaneous TLS and were subsequently diagnosed with T-cell malignancies.
Case presentation
Case 1
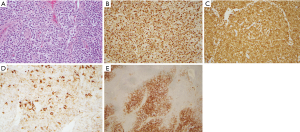
A 78 year-old African American man was seen in oncology clinic complaining of progressively worsening shortness of breath over the past 2 years, 30-pound weight loss over 3 months, and intermittent enlargement of groin lymph nodes. His medical history was significant for chronic obstructive pulmonary disease, chronic kidney disease, prostate cancer (status post-resection), hypertension and hyperlipidemia. On physical examination, he was afebrile (oral temperature: 98.1 F) with blood pressure of 111/61 mmHg, heart rate of 95 beats per minute, respiratory rate of 17 per minute and oxygen saturation 98% (on room air). Examination showed bilateral non-tender rubbery enlarged cervical, axillary, and inguinal lymph nodes and a spleen tip 6 cm below the costal margin. PET/Computed tomography showed markedly enlarged pericardiophrenic, para-esophageal, celiac, mesenteric, para-aortic and aorto-caval lymph nodes, and splenomegaly. Bone marrow biopsy and an excisional biopsy of the right inguinal lymph node were performed. Histopathology of the bone marrow showed increased cellularity involved by 10% of atypical lymphocytes forming small aggregates with CD3+ and CD45− phenotype (Figure 1). Lymph node biopsy showed effaced nodal architecture, increased cellular infiltrate with reactive lymphocytes, plasma cells, eosinophils and histocytes (Figure 2). A Ki-67 proliferative index of 90% (Figure 2B) and increased vascularity on H&E and CD34 staining were seen. Immunohistochemistry demonstrated strongly positive CD3 (cytoplasmic) expression alongside aggregates of CD20 and CD21 cells as markers of B-lymphocyte and follicular cells, respectively (Figure 2B,C,D,E). Immunophenotype was cCD3+, CD4+, CD5+, CD7+, Bcl-2+, CD30+ (partial), CD99+, and negative for CD1a. Pax5, CD45, TdT, and Alk1, weakly positive for PD-1 and CD10. EBER was positive for B cells. Flow cytometry studies showed a CD4 T-cell phenotype with cCD3, CD4, CD5, CD7, CD2, CD99 positive, CD1a, CD3, CD34, CD8, CD45, CD56, CD16 negative. Antibodies for HTLV1-II were negative. These features were consistent with mature T-cell lymphoma favoring a final diagnosis of angioimmunoblastic T-cell lymphoma. The patient was admitted to start treatment and his laboratory studies showed serum potassium of 6.0 mEq/dL, phosphorus 6.0 mg/dL, LDH 781 U/L, creatinine 3.1 mg/dL, uric acid 16.8 mg/dL, serum calcium level 8.5 mg/dL, and albumin 3.6 gm/dL. He was transferred to the medical intensive care unit and started on continuous veno-venous hemofiltration. The patient recovered and was given dose-adjusted EPOCH, a regimen consisting of etoposide, prednisolone, vincristine, cyclophosphamide and doxorubicin. The chemotherapy regimen was well tolerated and the patient had a complete response on follow-up evaluations as shown by clinical, PET imaging (Figure 3A,B), and bone marrow examination.


Case 2
A 49-year-old gentleman presented to the emergency department with complaints of subjective fever, night sweats, cough, and shortness of breath for 2 days. His past medical history was notable for hypertension, which was well-controlled on oral antihypertensive agents. His family history was strongly positive for malignancies in first-degree relatives (sister had gastric cancer, mother had breast cancer, and father had prostate cancer). On physical examination, patient was tachycardiac (pulse 102 beats per minute; regular), tachypneic (respiratory rate of 28 breaths per minute), and with prominent scleral icterus. Right submandibular, posterior cervical and supraclavicular lymph nodes were enlarged, non-tender, and rubbery in consistency. Bilateral inguinal lymphadenopathy and splenomegaly were also appreciated. Laboratory investigations were remarkable for anemia (hemoglobin: 7.7 g/dL; reference range: 13.5–17.5 g/dL), leukopenia (total leukocyte count: 0.7×109 cells/L; reference range: 4×109–11×109 cells/L), thrombocytopenia (platelet count: 70×109 cells/L), hyperkalemia (potassium: 5.6 mEq/L; reference range: 3.5–5.1 mEq/L), hyperphosphatemia (phosphorus: 4.7 mg/dL; reference range: 2.5–4.0 mg/dL), hypocalcemia (calcium: 7.6 mg/dL; reference range: 8.4–10.2 mg/dL), hyperuricemia (uric acid: 11.1 mg/dL; reference range: 3.4–7.0 mg/dL) and acute kidney injury (creatinine: 5.0 mg/dL; reference range: 0.6–1.1 mg/dL). Computed tomography of the chest, abdomen, and pelvis revealed splenomegaly with multiple splenic infarcts and bilateral axillary, mediastinal, left hilar, para-aortic, and left iliac lymphadenopathy. Based on the patient’s clinical presentation, a diagnosis of lymphoid malignancy complicated by spontaneous TLS was suspected. Patient was admitted to the intensive care unit and started on intermittent hemodialysis. Patient underwent bone marrow and left inguinal lymph node biopsy. Histopathological examination of the lymph node revealed diffuse lymphoid proliferation of medium- to large-sized atypical cells with irregular nuclei, dispersed chromatin and some mitoses. These atypical cells were strongly positive for CD3, CD34 and TdT; and weakly positive for CD1a, CD10 and CD79a. Flow cytometry of the lymph node tissue revealed 60% T lymphoblasts with the following immunophenotype: CD2–, CD3–, cCD3+, CD5+ (dim), CD7+, CD4–, CD8–, CD33+, CD34+ (partial), TdT+ (partial), CD19–, cCD79a+ (dim) and MPO–. Flow cytometry of the bone marrow aspirate showed 9% T lymphoblasts, while core biopsy revealed normocellular bone marrow for age with 50–60% involvement by T lymphoblasts. Based on these histopathological findings, a diagnosis of T-cell acute lymphoblastic leukemia was made. After a few sessions of hemodialysis, patient’s laboratory investigations improved and he was transferred to a specialized cancer center for further evaluation and management.
Discussion
TLS is an oncologic emergency associated with a constellation of characteristic laboratory abnormalities viz. hyperkalemia, hyperphosphatemia, hypocalcemia, and hyperuricemia (1). These metabolic derangements are a consequence of massive turnover of malignant cells with release of intracellular contents into the bloodstream. If left untreated, TLS can progress further and result in fatal complications such as acute kidney injury, cardiac arrhythmias, seizures, uremia, and even death (2). Cairo and Bishop devised criteria for the diagnosis of clinical TLS and laboratory TLS in 2004 (4). They defined laboratory TLS as derangement in serum values of two or more of the following by 25% from baseline within 3 days prior to, or 7 days after initiation of chemotherapy: uric acid, potassium, phosphorus, and/or calcium. Cairo-Bishop definition of clinical TLS requires the presence of laboratory TLS along with any one of the following, provided there is no alternative explanation: (I) increase in serum creatinine to 1.5 times the upper limit of normal for age; (II) cardiac arrhythmia or sudden cardiac death; or (III) seizures. Both of our cases meet the Cairo-Bishop definition of clinical TLS. TLS is more commonly seen after treatment of hematological malignancies such as acute leukemia and non-Hodgkin lymphoma and its incidence is generally decreased with use of preventive measures.
Importantly, the development of TLS has a significant impact on patient’s prognosis as it can hinder the administration of highly effective chemotherapy and increase risk of morbidity and mortality (5). Using data from the Healthcare Cost and Utilization Project’s Nationwide Inpatient Sample, Candrilli and colleagues reported that acute renal failure and receipt of renal replacement therapy among patients with hematologic malignancies increased the mean length of hospital stay from 7.4 to 17.6 days and nearly doubled the cost of hospitalization from $13,947 to $44,619 USD (6). In the two cases reported herein, the length of hospital stay was 10 and 7 days respectively.
TLS often develops following treatment of aggressive malignancies with intensive traditional chemotherapy. However, there are case reports illustrating that TLS can also occur independently of chemotherapy due to the use of weak tumoricidal like glucocorticoids, as well as targeted monoclonal therapies and radiation treatments (7-9). An international TLS expert panel developed a model of risk stratification for patients with malignancies and made recommendations for TLS prophylaxis in 2010 (5). For patients deemed at high risk for TLS, frequent laboratory monitoring is recommended along with aggressive intravenous hydration (3 L/m2 of body surface area) and a single dose of rasburicase (0.1–0.2 mg/kg), which may be repeated only if clinically necessary. In patients with G6PD def, rasburicase can be substituted for allopurinol. Allopurinol is a sulfa drug that inhibits xanthine oxidase and reduces the production of uric acid. On the other hand, rasburicase is a recombinant uric acid oxidase that uses uric acid as a substrate to produce allantoin—a harmless end-product of uric acid metabolism that is excreted inertly in the urine (10). Intravenous hydration promotes urinary flow and reduces the likelihood of renal tubular obstruction (1,2). Alkalinization of urine is not recommended by the international TLS expert panel’s guidelines (5). Both cases reported herein would fall in the high risk category for TLS as per the expert panel’s risk stratification model.
Renal replacement therapy is often required in patients with TLS who develop acute oliguric renal failure for efficient removal of solutes (11). Oliguria due to TLS often responds fairly rapidly to renal replacement therapy as purine by-products, uric acid, phosphorus, and potassium are cleared from the blood. Hemodialysis primarily relies on the removal of solutes by diffusion and the rate of removal is governed by Fick’s law of diffusion. On the other hand, ultrafiltration removes solutes by convection (solvent drag) and is heavily dependent on the rate of ultrafiltration. In patients with TLS, no study has directly compared intermittent hemodialysis, continuous veno-venous hemofiltration, continuous veno-venous hemodialysis and continuous veno-venous hemodiafiltration. Previously published reports suggest that all of these modalities of renal replacement therapy have similar efficacy (1,2,11). In the two cases we described, continuous veno-venous hemodialysis and intermittent hemodialysis were employed respectively with a favorable outcome in both cases.
Spontaneous TLS is a rare phenomenon that has been well-described in patients with hematologic malignancies (12), although it can occur in patients with solid tumors as well (5). Among solid tumors, spontaneous TLS has been reported to occur in melanoma, neuroblastoma, small cell lung cancer, and a variety of other cancers (13). In the two cases we report, spontaneous TLS was the first manifestation of an underlying T-cell lymphoid malignancy (viz. acute lymphoblastic leukemia and angioimmunoblastic lymphoma). This is in line with previously reported cases where spontaneous TLS can be the first manifestation of an undiagnosed hematologic or solid malignancy (3,10,13). Interestingly, certain cases have shown that hyperphosphatemia and hypocalcemia occur less frequently with spontaneous TLS. Thus making it more challenging to recognize certain cases of spontaneous TLS and underestimating the true incidence and severity of this syndrome (14,15). Consequently, there is an important clinical question of whether the currently accepted diagnostic criterion is appropriate to recognize spontaneous TLS. Reports have shown that some patients with spontaneous TLS do not meet all diagnostic criteria and, to this end, certain studies have proposed a diagnosis of spontaneous TLS as acute renal failure with elevated lactate dehydrogenase, uric acid nephropathy in setting of suspicion or proven malignancy, rather than focusing on fulfilling metabolic derangements (14,16). Furthermore, these studies suggested that the diagnosis of spontaneous TLS should be highly considered if a patient’s clinical presentation is suspicious of an undiagnosed malignancy with laboratory findings significant for increased cell turnover causing end organ damage. This approach, although simplistic, could help increase earlier diagnosis and minimize morbidity and mortality associated with undiagnosed spontaneous TLS. This is of considerable importance for all clinicians in general, and emergency physicians in particular, as timely recognition of TLS and appropriate management can be lifesaving.
Conclusions
Spontaneous TLS is a rare clinical entity that may be seen in patients with rapidly dividing liquid or solid malignancies. Given that spontaneous TLS can be the first manifestation of an underlying malignancy, all physicians should be familiar with this oncologic emergency and have a high index of suspicion. Early recognition and prompt management can be lifesaving for patients with an otherwise curable malignancy.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images. The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Gilbert S, Wright S. Tumor lysis syndrome. In: Jhaveri K, Salahudeen A. editors. Onconephrology. New York, NY: Springer Publishing, 2015:163-81.
- Koneru H, Bozyk PD. Tumor lysis syndrome. In: Hyzy R. editor. Evidence-Based Critical Care. New York, NY: Springer Publishing, 2017:641-5.
- Takeuchi N, Miyazawa S, Ohno Z, et al. A case of spontaneous tumor lysis syndrome in malignant melanoma. World J Oncol 2016;7:40-4. [Crossref] [PubMed]
- Cairo MS, Bishop M. Tumour lysis syndrome: new therapeutic strategies and classification. Br J Haematol 2004;127:3-11. [Crossref] [PubMed]
- Cairo MS, Coiffier B, Reiter A, et al. Recommendations for the evaluation of risk and prophylaxis of tumour lysis syndrome (TLS) in adults and children with malignant diseases: an expert TLS panel consensus. Br J Haematol 2010;149:578-86. [Crossref] [PubMed]
- Candrilli S, Bell T, Irish W, et al. A comparison of inpatient length of stay and costs among patients with hematologic malignancies (excluding Hodgkin disease) associated with and without acute renal failure. Clin Lymphoma Myeloma 2008;8:44-51. [Crossref] [PubMed]
- Dhakal P, Rai MP, Thrasher M, et al. Spontaneous tumor lysis syndrome in small cell lung cancer: a rare phenomenon. BMJ Case Rep 2018;2018.
- Tosi P, Barosi G, Lazzaro C, et al. Consensus conference on the management of tumor lysis syndrome. Haematologica 2008;93:1877-85. [Crossref] [PubMed]
- Belay Y, Yirdaw K, Enawgaw B. Tumor lysis syndrome in patients with haematological malignancies. J Oncol 2017;2017:9684909. [Crossref] [PubMed]
- Gbaguidi X, Goodrich L, Roca F, et al. Bulky solid tumors in elderly adults: beware of spontaneous tumor lysis syndrome. J Am Geriatr Soc 2016;64:235-7. [Crossref] [PubMed]
- Malik IA, Abubakar S, Alam F, et al. Dexamethasone-induced tumor lysis syndrome in high-grade non-Hodgkin’s lymphoma. South Med J 1994;87:409-11. [Crossref] [PubMed]
- Yang H, Rosove MH, Figlin RA. Tumor lysis syndrome occurring after the administration of rituximab in lymphoproliferative disorders: high-grade non-Hodgkin’s lymphoma and chronic lymphocytic leukemia. Am J Hematol 1999;62:247-50. [Crossref] [PubMed]
- Dar L, Gendelman O, Amital H. Tumor lysis syndrome presenting in a patient with metastatic melanoma treated with radiation therapy. Isr Med Assoc J 2014;16:456-7. [PubMed]
- Hsu HH, Chan YL, Huang CC. Acute spontaneous tumor lysis presenting with hyperuricemic acute renal failure: clinical features and therapeutic approach. J Nephrol 2004;17:50-6. [PubMed]
- Alkhuja S, Ulrich H. Acute renal failure from spontaneous acute tumor lysis syndrome: a case report and review. Ren Fail 2002;24:227-32. [Crossref] [PubMed]
- Weeks AC, Kimple ME. Spontaneous Tumor Lysis Syndrome: A Case Report and Critical Evaluation of Current Diagnostic Criteria and Optimal Treatment Regimens. J Investig Med High Impact Case Rep 2015;3:2324709615603199. [Crossref] [PubMed]
Cite this article as: Roque W, Rehman A, Suero-Abreu GA, Danek BA, Colao J, Fayngersh A, Srinivas S, Kra J, Cai D, Chang VT. Spontaneous tumor lysis syndrome in T-cell malignancy: two case reports. Stem Cell Investig 2019;6:24.


