
Engineered platelets with antithrombotic and antimetastatic properties
Platelets are small anucleate blood cells that originally derive from bone marrow megakaryocytes from hematopoietic stem cells through a process known as megakaryopoiesis (1,2). They play an indispensable role in normal hemostasis and thrombosis after injury. When blood vessels are damaged, platelets are exposed to the basement membrane and collagen at the wounding site, rapidly triggering platelet activation and aggregation. Activated platelets change shape and release various cytokines, growth factors, and other related molecules to recruit additional platelets and immune cells along with initiating the coagulation cascade and thrombogenesis. Subsequently, firm blood clots are formed, which can prevent bleeding and promote healing (3,4). However, increasing evidence has indicated that in addition to physiological functions, highly activated platelets contribute to abnormal blood coagulation and thrombosis under pathological circumstances including tumor development as well as cerebrovascular disease and cardiovascular disorders. It is generally accepted that tumors behave as chronic or non-healing wounds while platelets act as the first responders in tumor metastasis as well as wound healing. Cancer patients with increased platelets are at higher risk of thrombosis (5) and have a worse prognosis and shorter survival (6-10). From clinical data, thrombosis is the second leading cause of malignancy-associated death (11). Therefore, targeting platelets may be a useful adjuvant strategy for platelet-related disease therapy. So far, multiple antiplatelet agents including aspirin, abciximab, clopidogrel, prasugrel, and ticagrelor have been developed (12). Unfortunately, use of these drugs tends to cause excessive reduction of platelet function. Considering that production of new platelets takes at least 7 to 10 days, patients who take antiplatelet agents are at the high risk of bleeding if the antiplatelet effects cannot be reversed in time. Therefore, it would be ideal to keep the beneficial healing properties of platelets and avoid their prothrombotic and protumorigenic damaging effects.
In a recent report published in Science Translational Medicine (13), Papa and colleagues have inventively generated platelet decoys from natural healthy human platelets through detergent extraction and successfully developed a controllable antiplatelet therapy in preclinical models (Figure 1). They first isolated platelets by centrifugation and then treated the platelets using a buffer containing the detergent Triton X-100. This resulted in removal of most membranes and intracellular contents but retained most cell surface proteins in the normal position due to an intact cytoskeleton. Compared to normal platelets, the platelet decoys exhibited a similar round shape but had smaller size and reduced granularity with decreased membrane adhesion receptors. They did not activate and aggregate with platelet agonists under physiological conditions. Interestingly, adding platelet decoys (D) to intact platelets (P) at a ratio of 1:5 (D: P) reduced aggregation responses of platelets under the stimulation of ADP and collagen, showing similar antiplatelet activity of clinically approved antithrombotic drugs aspirin and abciximab. This result indicated that decoys may interfere with interactions between functional platelets. Encouragingly, decoys neither disrupted the thrombin activation pathway and normal hemostasis nor caused damage to the liver and spleen tissues.
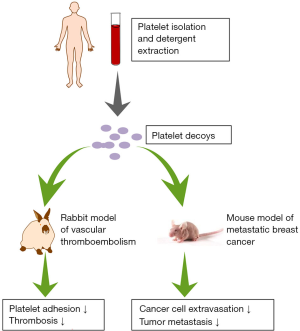
Subsequent experiments demonstrated that though platelet decoys did not adhere to extracellular matrix (ECM) substrates themselves, they reduced native platelet adhesion to thrombogenic surfaces, implying that decoys indeed disturbed the functions of platelets. Furthermore, platelet decoys inhibited blood clot formation in a rabbit thromboembolic model which had similar circulating platelet count and cell size distribution as humans. More importantly, the inhibitory effects of platelet decoys could be reversed immediately by addition of 20% fresh platelets. Currently, antiplatelet activity of clinically used antiplatelet drugs cannot be alleviated through platelet transfusion, putting treated patients with hemorrhage in life-threatening danger. Therefore, platelet decoys are likely to be a promising treatment alternative.
Since platelets can adhere to circulating cancer cells (CTCs) and protect CTCs in metastatic cascade (14), Papa et al. have explored the effects of platelet decoys on cancer cells. In a mouse model of breast cancer, they found that decoys could effectively bind to breast cancer cells mainly through GPIIb/IIIa receptors and decrease cancer cell arrest and extravasation. Though metastatic tumor growth was not affected in the presence of natural platelets or decoys alone, preincubation of decoys and platelets at a ratio of 1:5 or 2:5 exhibited dose-dependent inhibition of metastatic tumor load. Higher D: P ratio led to more significant reduction in tumor mass, demonstrating that platelet decoys disturbed the functional platelet-cancer cell interaction. Nevertheless, whether platelet decoys take effect in other types of tumors aside from breast cancer needs to be investigated.
In short, Papa et al. developed platelet decoys as a reversible, drug-free, cell-based, antiplatelet therapy which may be beneficial to treat patients with thrombotic disorders or metastatic tumors. In contrast to current antiplatelet drugs, platelet decoys possess greater benefits as they are a fast-acting cellular therapeutic approach and their inhibitory effects can be reversed simply by transfusing functional platelets. As decoys lose their granule contents, they are possibly unable to release related molecules to amplify the activation response or initiate receptor-mediated signaling pathways, though they retain a certain number of membrane adhesion receptors. As a consequence, decoys produce antiplatelet effects and thus block thrombosis and tumor metastatic cascade through competing with intact platelets for binding sites on platelets or cancer cells.
Given that platelet decoys have preventive effects on thrombosis and cancer dissemination, platelet decoys could be applied as an auxiliary treatment after cardiovascular injuries, thrombolytic therapy, or tumor resection. It should be noted that decoys from a patient should be transfused into the same patient to reduce immunological rejection. Additionally, decoys have the potential to be drug delivery vehicles like platelets (15), targeting thrombi and tumors. They may act in synergy with the carried drug and play a much stronger role in inhibition of blood clot formation and tumor metastasis. However, there is still a long way to go in clinical use of platelet decoys. First of all, it is important to confirm the circulation time of platelet decoys. If decoys are cleared out more rapidly than natural platelets, researchers need to boost the longevity of decoys for better application. Since the key step in the generation of platelet decoys is detergent extraction, types and concentrations of detergents and extraction time and conditions can be further optimized. The integrity and stability of platelet decoys may be improved by the limited fixation. In addition, identifying components responsible for the beneficial and damaging effects of platelets also helps to find other strategies to generate better platelet decoys. Second, since Papa et al. conducted this study in immune-deficient models with human platelet-derived decoys, it is necessary to validate the antiplatelet activity of animal-derived platelet decoys transfused into the same animal to exclude the influence caused by species difference. However, experimental results from animal-derived decoys may have little clinical significance because the ultimate clinical product will be human-derived platelets. To detect the immunogenicity of human-derived platelet decoys more accurately before clinical trials, studies should be carried out in animals with high affinity to humans, such as monkeys. Furthermore, animal experiments could be conducted to determine effective dose and NOAEL (no observed adverse effect level) of human-derived platelet decoys. Moreover, long-term toxicological testing may be helpful to assess the influence of platelet decoys on multiple organs, especially organs associated with hematopoiesis, including the lung (16), liver, spleen and borrow marrow. After preclinical studies, many clinical trials will be required to verify the appropriate dose, safety, and effectiveness of decoys in patients.
References
- Guo T, Wang X, Qu Y, et al. Megakaryopoiesis and platelet production: insight into hematopoietic stem cell proliferation and differentiation. Stem Cell Investig 2015;2:3. [PubMed]
- Semple JW, Italiano JE Jr, Freedman J. Platelets and the immune continuum. Nat Rev Immunol 2011;11:264-74. [Crossref] [PubMed]
- Agbani EO, Poole AW. Procoagulant platelets: generation, function, and therapeutic targeting in thrombosis. Blood 2017;130:2171-9. [Crossref] [PubMed]
- Menter DG, Kopetz S, Hawk E, et al. Platelet "first responders" in wound response, cancer, and metastasis. Cancer Metastasis Rev 2017;36:199-213. [Crossref] [PubMed]
- Timp JF, Braekkan SK, Versteeg HH, et al. Epidemiology of cancer-associated venous thrombosis. Blood 2013;122:1712-23. [Crossref] [PubMed]
- Cheng J, Zeng Z, Ye Q, et al. The association of pretreatment thrombocytosis with prognosis and clinicopathological significance in cervical cancer: a systematic review and meta-analysis. Oncotarget 2017;8:24327-36. [PubMed]
- Long Y, Wang T, Gao Q, et al. Prognostic significance of pretreatment elevated platelet count in patients with colorectal cancer: a meta-analysis. Oncotarget 2016;7:81849-61. [Crossref] [PubMed]
- Wang YH, Kang JK, Zhi YF, et al. The pretreatment thrombocytosis as one of prognostic factors for gastric cancer: A systematic review and meta-analysis. Int J Surg 2018;53:304-11. [Crossref] [PubMed]
- Zhang X, Ran Y. Prognostic role of elevated platelet count in patients with lung cancer: a systematic review and meta-analysis. Int J Clin Exp Med 2015;8:5379-87. [PubMed]
- Schlesinger M. Role of platelets and platelet receptors in cancer metastasis. J Hematol Oncol 2018;11:125. [Crossref] [PubMed]
- Khorana AA, Francis CW, Culakova E, et al. Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. J Thromb Haemost 2007;5:632-4. [Crossref] [PubMed]
- Ji X, Hou M. Novel agents for anti-platelet therapy. J Hematol Oncol 2011;4:44. [Crossref] [PubMed]
- Papa AL, Jiang A, Korin N, et al. Platelet decoys inhibit thrombosis and prevent metastatic tumor formation in preclinical models. Sci Transl Med 2019. [Crossref] [PubMed]
- Li N. Platelets in cancer metastasis: To help the "villain" to do evil. Int J Cancer 2016;138:2078-87. [Crossref] [PubMed]
- Lu Y, Hu Q, Jiang C, Gu Z. Platelet for drug delivery. Curr Opin Biotechnol 2019;58:81-91. [Crossref] [PubMed]
- Lefrançais E, Ortiz-Muñoz G, Caudrillier A, et al. The lung is a site of platelet biogenesis and a reservoir for haematopoietic progenitors. Nature 2017;544:105-9. [Crossref] [PubMed]
Cite this article as: Yu L, Zhao ZJ. Engineered platelets with antithrombotic and antimetastatic properties. Stem Cell Investig 2019;6:27.


