
Lower T cell inhibitory receptor level in mononuclear cells from cord blood compared with peripheral blood
Introduction
It is well known that umbilical cord blood (CB) cells have been used as one of standard source of hematopoietic stem cells (HSC) for allogeneic HSC transplantation (HSCT), and also offered an attractive, allogeneic T cell and natural killer (NK) cell source for immunotherapy such as chimeric antigen receptor (CAR)-T cells therapy, and increased their potential clinical applications for different disease such as ischemic stroke and anti-inflammation, etc. (1-4). T cell immunity of CB is relative different from peripheral blood (PB), which was characterized by different T cell repertoire, naive T cell number, T cell activation etc. (5-6). The characteristics of negative co-stimulator signal transduction pathway in CB T cells remains unclear. Recent reports showed that T cell inhibitory receptor (immunosuppressive receptor) such as programmed death-1 (PD-1), cytotoxic T lymphocyte antigen-4 (CTLA-4), T cell immunoglobulin mucin-3 (Tim-3), B and T lymphocyte attenuator (BTLA) play important roles in immune homeostasis (7). Overexpression of these factors involved in tumor immunosuppression and is associated with poor prognosis in cancer as well as hematological malignancy patients, while downregulation of these factors may be related to an autoimmune disorder. For example, upregulating PD-1 was found in T cells in PB from patients with hematological malignancies, while downregulating PD-1 was detected in patients with immune thrombocytopenic purpura (ITP) (7,8-10). It is well known low immunogenicity in CB T cells, while CB T cells showed higher potency of activation and proliferation after cytokine and antigen stimulation (11). Whether this feature is associated with T cell inhibitory receptor expression, in this study, we analyzed the expression levels of PD-1, CTLA-4, Tim-3, lymphocyte activation gene-3 (LAG-3) and BTLA genes in CB compared with PB.
Methods
Samples
Umbilical CB samples were collected from 15 full-term healthy babies at the delivery. PB from 22 healthy individual (12 males and 10 females; median age: 40 years; range, 21–71 years) were served as control. All of the experiments were conducted with the understanding and the consent of each participant, and ethical approval was obtained from the Ethics Committee of First Affiliated Hospital, School of Medicine, Jinan University.
Analysis of PD-1, CTLA-4, Tim-3, LAG-3 and BTLA gene expression
Mononuclear cells from CB and PB were isolated, According to the manufacturer’s protocol, RNA was isolated, and then cDNA synthesis was performed, The expression levels of PD-1, CTLA-4, Tim-3, LAG-3, BTLA and the β2-microglobulin (β2-MG) reference gene (the sequences of primers were listed in Table 1) were detected by SYBR Green I quantitative real-time reverse transcription-polymerase chain reaction (qRT-PCR) as previously described (12).
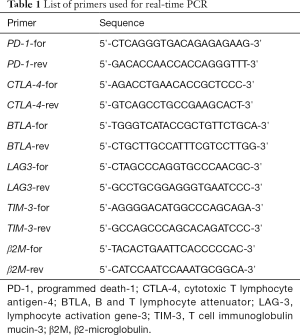
Full table
Statistical analyses
The Mann-Whitney test was used for analysis the differences of gene expression between CB and PB groups. Data are represented by medians. Spearman’s rank correlation test was used to analyze the expression levels of all of the detected genes in this study. Differences were considered statistically significant with a P<0.05.
Results and discussion
The expression levels of all five T cell inhibitory receptor genes, PD-1, CTLA-4, Tim-3, LAG-3 and BTLA were compared between 15 cases of CB samples and PB samples from 22 cases. Significantly, the expression level of PD-1 (median: 0.002, P=0.000), CTLA-4 (median: 0.001, P=0.000), LAG-3 (median: 0.023, P=0.000) and BTLA (median: 0.85, P=0.000) were lower in CB group compared with PB group (Figure 1A,B,C,D). While the expression level of Tim-3 was similar between CB and PB (median: 1.791 vs. 1.614, P=0.951) group (Figure 1E). Interesting, expression tendency of PD-1, CTLA-4, LAG-3 and BTLA genes looks paralleled between CB and PB (Figure 1F,G). We further analyzed the correlation of expression levels between the five genes respectively, positive correlation of gene expression levels was found between PD-1 and CTLA-4, PD-1 and LAG-3, CTLA-4 and LAG-3 respectively (Figure 2). The remains had no significant correlation. While, positive correlation was found only between LAG-3 and TIM-3 in healthy individuals. Overall, the findings indicated that lower expression of most T cell inhibitory receptors is one characteristic in CB, this provide a novel information for the immunotherapy by using CB T cells. This may be due to higher numbers of naive T cells and non-activation status of T cells in CB (5). And it seems not complementary of expression regulation between different T cell inhibitory receptor genes. However, it is interesting, why there is not different in the expression level of TIM-3 between CB and PB? It is needed further investigation.
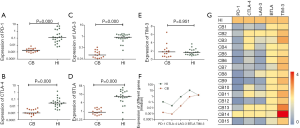

There are few studies regarding expression of T cell inhibitory receptors in CB. Most studies focused to characterize the distinctive nature of CB T cells after stimulation, which is expected to explain the reason related to graft-vs-host disease (GVHD) or disease relapse after HSCT, etc. For example, reduced surface and intracellular expression of CTLA-4 in stimulated (with anti-CD3 and anti-CD28 monoclonal antibodies) CB T cells were detected in comparison with adult PB controls. Inhibiting CLTA-4 expression in CB T cells may contribute to favorable CB T cell allogeneic responses (13). Increasing PD-1 expression on CD4+ T cells is associated with increased risk for mortality allogeneic HSCT, especially with CB transplantations (14). While significantly higher number of PD-1+ CD8+ T cells was detected in pediatric patients who subsequently experienced leukemic relapse in CB stem cell transplantation (SCT) (15). Whether these results are related to the original feature of lower T cell inhibitory receptor expression in CB T cells, it remains an open question. It may be worthy to dynamically detect and try to find out the range of different expression level and the change of the T cell inhibitory receptor expression pattern in CB T cells before and after transplantation, which may explain the reason. Moreover, increasing data showed that CAR-T cells had made great successfully for implementation of cellular immunotherapy for high-risk leukemia and lymphoma patients (16-20). However, T cells from patients with leukemia and lymphoma which expose in leukemia bone marrow or tumor microenvironment, acquire a senescent and exhausted phenotype, leading to a progression towards terminal differentiation (10,21,22). Particularly, upregulation of T cell inhibitory receptors like PD-1 and Tim-3, etc. results to significantly inhibit T cell activation, this may influence the CAR-T function which are produced from such patients T cells, it might have less effectiveness to attack leukemia and lymphoma cells (18,23,24). Thus, allogeneic derived CAR-T cells may avoid this barrier. And CB are one of attractive, allogeneic T cell and NK cell source for a novel approach to immunotherapy using engineered CB-derived T cells or NK cells (3,4), in particularly, in this study, we showed that low expression of T cell inhibitory receptors may support its advantage as source for CAR-T production.
Conclusions
We firstly characterized the expression profile of PD-1, CTLA-4, Tim-3, LAG-3 and BTLA in CB, and concluded their different expression pattern from PB. The significance on lower level of such T cell inhibitory receptor in CB is worthy to further discuss their role on T cell immune response when CB is used in SCT as well as in cellular immunotherapy.
Acknowledgments
Funding: This study was supported by grants from the National Natural Science Foundation of China (No. 81570143, 91642111 and 81770152) and the Guangzhou Science and Technology Project (No. 201510010211, 201807010004, 201803040017).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the Ethics Committee of the first affiliated hospital of Jinan University {No. [2015]009}. All of the experiments were conducted with the understanding and the consent of each participant.
References
- Xu L, Chen H, Chen J, et al. The consensus on indications, conditioning regimen, and donor selection of allogeneic hematopoietic cell transplantation for hematological diseases in China-recommendations from the Chinese Society of Hematology. J Hematol Oncol 2018;11:33. [Crossref] [PubMed]
- Ma Q, Garber HR, Lu S, et al. A novel TCR-like CAR with specificity for PR1/HLA-A2 effectively targets myeloid leukemia in vitro when expressed in human adult peripheral blood and cord blood T cells. Cytotherapy 2016;18:985-94. [Crossref] [PubMed]
- Liu E, Tong Y, Dotti G, et al. Cord blood NK cells engineered to express IL-15 and a CD19-targeted CAR show long-term persistence and potent antitumor activity. Leukemia 2018;32:520-31. [Crossref] [PubMed]
- Huang L, Liu Y, Lu J, et al. Intraarterial transplantation of human umbilical cord blood mononuclear cells in hyperacute stroke improves vascular function. Stem Cell Res Ther 2017;8:74. [Crossref] [PubMed]
- Li Y, Chen S, Yang L, et al. TRAV and TRBV repertoire, clonality and the proliferative history of umbilical cord blood T-cells. Transpl Immunol 2007;18:151-8. [Crossref] [PubMed]
- Cantó E, Rodríguez-Sánchez JL, Vidal S. Naive CD4+ cells from cord blood can generate competent Th effector cells. Transplantation 2005;80:850-8. [Crossref] [PubMed]
- Zhong J, Chen S, Xu L, et al. Lower expression of PD-1 and PD-L1 in peripheral blood from patients with chronic ITP. Hematology 2016;21:552-7. [Crossref] [PubMed]
- Alfayez M, Borthakur G. Checkpoint inhibitors and acute myelogenous leukemia: promises and challenges. Expert Rev Hematol 2018;11:373-89. [Crossref] [PubMed]
- Tan J, Chen S, Huang J, et al. Increased exhausted CD8+ T cells with programmed death-1, T-cell immunoglobulin and mucin-domain-containing-3 phenotype in patients with multiple myeloma. Asia Pac J Clin Oncol 2018;14:e266-74. [Crossref] [PubMed]
- Tan J, Chen S, Lu Y, et al. Higher PD-1 expression concurrent with exhausted CD8+ T cells in patients with de novo acute myeloid leukemia. Chin J Cancer Res. 2017;29:463-70. [Crossref] [PubMed]
- Frumento G, Zheng Y, Aubert G, et al. Cord blood T cells retain early differentiation phenotype suitable for immunotherapy after TCR gene transfer to confer EBV specificity. Am J Transplant 2013;13:45-55. [Crossref] [PubMed]
- Liao Z, Lv X, Liu S, et al. Different aberrant expression pattern of immune checkpoint receptors in patients with PTCL and NK/T-CL. Asia Pac J Clin Oncol 2018;14:e252-8. [Crossref] [PubMed]
- Miller RE, Fayen JD, Mohammad SF, et al. Reduced CTLA-4 protein and messenger RNA expression in umbilical cord blood T lymphocytes. Exp Hematol 2002;30:738-44. [Crossref] [PubMed]
- Schade H, Sen S, Neff CP, et al. Programmed Death 1 Expression on CD4+ T Cells Predicts Mortality after Allogeneic Stem Cell Transplantation. Biol Blood Marrow Transplant 2016;22:2172-9. [Crossref] [PubMed]
- Merindol N, Champagne MA, Duval M, et al. CD8(+) T-cell reconstitution in recipients of umbilical cord blood transplantation and characteristics associated with leukemic relapse. Blood 2011;118:4480-8. [Crossref] [PubMed]
- Lichtenegger FS, Krupka C, Haubner S, et al. Recent developments in immunotherapy of acute myeloid leukemia. J Hematol Oncol 2017;10:142. [Crossref] [PubMed]
- Wei G, Ding L, Wang J, et al. Advances of CD19-directed chimeric antigen receptor-modified T cells in refractory/relapsed acute lymphoblastic leukemia. Exp Hematol Oncol 2017;6:10. [Crossref] [PubMed]
- Fan M, Li M, Gao L, et al. Chimeric antigen receptors for adoptive T cell therapy in acute myeloid leukemia. J Hematol Oncol 2017;10:151. [Crossref] [PubMed]
- Tasian SK. Acute myeloid leukemia chimeric antigen receptor T-cell immunotherapy: how far up the road have we traveled? Ther Adv Hematol 2018;9:135-48. [Crossref] [PubMed]
- Rossi J, Paczkowski P, Shen YW, et al. Preinfusion polyfunctional anti-CD19 chimeric antigen receptor T cells are associated with clinical outcomes in NHL. Blood 2018;132:804-14. [Crossref] [PubMed]
- Ye B, Stary CM, Li X, et al. Engineering chimeric antigen receptor-T cells for cancer treatment. Mol Cancer 2018;17:32. [Crossref] [PubMed]
- Yao D, Xu L, Tan J, et al. Re-balance of memory T cell subsets in peripheral blood from patients with CML after TKI treatment. Oncotarget 2017;8:81852-9. [Crossref] [PubMed]
- Cherkassky L, Morello A, Villena-Vargas J, et al. Human CAR T cells with cell-intrinsic PD-1 checkpoint blockade resist tumor-mediated inhibition. J Clin Invest 2016;126:3130-44. [Crossref] [PubMed]
- Guha P, Cunetta M, Somasundar P, et al. Frontline Science: Functionally impaired geriatric CAR-T cells rescued by increased α5β1 integrin expression. J Leukoc Biol 2017;102:201-8. [Crossref] [PubMed]
Cite this article as: Lin Y, Lin J, Huang J, Chen Y, Tan J, Li Y, Chen S. Lower T cell inhibitory receptor level in mononuclear cells from cord blood compared with peripheral blood. Stem Cell Investig 2019;6:35.


