Adipose-derived stem cells and their microenvironment (Niche) in type 2 diabetes mellitus
Introduction
Type-2 diabetes mellitus (T2DM) is one of the major diseases in the world that causes death and characterized by insulin resistance and high blood glucose level. The prevalence of T2DM is continuously rising globally (1). Many studies proposed that T2DM is a metabolic disorder. At most cases, T2DM is related to obesity and associated with increased deposits of lipids in adipose tissue. Adipose tissue plays an important role in regulating energy and glucose homeostasis in systemic level, thus numerous studies on adipose tissue tried to discover the effect of obesity and metabolic disorder that affect the physiology (2).
In T2DM, adipose tissue becomes altered in function that may contribute to systemic inflammation. Inflammation in adipose tissue is associated with elevation of cytokine secretion and infiltration of leukocytes that induce apoptosis (2). Many strategies have been proposed to minimize the effect of type 2 diabetes, including treatment with cell therapy using mesenchymal stem cells (MSCs) either from adipose tissue or bone marrow (3). Recently, the use of stromal vascular fraction (SVF), which contains adipose stem cells (ASCs), has been proposed as an alternative therapy with a day care procedure for T2DM. The SVF consists of many types of cells including adherent and non-adherent cells when cultured.
Furthermore, SVF becomes popular due to the content of ASCs, which could be differentiated into many types of cells. However, the negative effect of T2DM on tissue microenvironment (niche) (4) might have an impact on the cells, including ASCs and various cells in SVF. Therefore, this review addresses the effect of T2DM on adipose tissue, including the main function of adipose tissue that includes adipogenesis, microenvironment of ASCs, and SVF in T2DM.
Adipogenesis
The main function of adipose tissue is to store the surplus energy in the form of neutral triglycerides (TGs) and to protect against cold. Adipose tissue consists of cells, namely adipocyte, pre-adipocytes, endothelial cells, pericytes, fibroblasts, macrophages, leucocytes, MSCs, hematopoietic stem cells (HSCs), as well as extracellular matrix (ECM) including collagen substrate (2,5). Adipose tissue can be classified into three types, i.e., white adipose tissue (WAT), brown adipose tissue (BAT) and perivascular adipose tissue (PVAT) (6).
WAT is a type of adipose tissue where the adipocytes contain one large lipid droplet for lipid storage, and a low number of mitochondrial content. This type of adipose tissue is found in subcutaneous or intra-abdominal area (6). In addition, WAT is also highly vascularized and has the highest expression of CD34 on its SVF (7). BAT, which is mainly found in subscapular area, has adipocytes that contain multiple lipid droplets, and a higher number of mitochondrial contents compared to adipocytes in WAT and PVAT. Perivascular adipose tissue, which is found around the blood vessels, contains adipocytes that have a higher number of lipid droplets and mitochondrial contents compared to adipocytes in WAT.
The variation of those types of adipose tissue occurs through adipogenesis. Adipogenesis is a process of cell differentiation from stem cell to adipocyte (8). When a stem cell such as ASCs undergoes differentiation, the stem cell will divide into two cells, where one cell keeps the stemness and the other cell differentiates into mature adipocyte through progenitor and pre-adipocyte differentiation process. Progenitor of adipocyte forms a mass of rounded, closely apposed cell while pre-adipocyte is an intermediate stage of adipocyte precursors generated from the differentiation of progenitor of adipocyte. Mature adipocytes are terminally-differentiated of adipocyte which form a rounded cell and store the surplus energy (9). ASCs differentiation into adipocytes is regulated by a specific microenvironment (4). Once a cell differentiated into an adipocyte, it showed high level of peroxisome proliferator-activated receptor γ (PPARγ) expression, which regulated adipogenesis (8,10). The stimulation of PPARɣ in adipose progenitor cell results in vascular endothelial growth factor receptor (VEGFR) and platelet-derived growth factor receptor beta (PDGFRβ) activation leading to extensive local vascular sprouting and increased vessel niche affinity (8). Overexpression of PPARγ induces adipogenesis, while the lack of PPARγ expression fails to promote adipogenesis. Interestingly, the role of PPARγ is not only to promote adipogenesis but also maintains the adipocyte lineage. In contrast, aberration of PPARγ protein leads to disturbed energy homeostasis and adipose tissue development. Those conditions increase the amount of lipid followed by increasing the size of adipocyte (hypertrophy), which induces a form of obesity-related disorder (10).
Microenvironment of ASCs
Microenvironment of cell (niche) refers to specific location where cells are found and interact with other cells or substrates (ECM), such as ASCs that are found in adipose tissue niche. The surrounding microenvironment of ASCs actively induces signals to promote either proliferation or differentiation. However, niche function is to protect ASCs from over activity of proliferation or differentiation (4). There are several important factors that regulate ASCs characteristics within the niche, where there are interaction between ASCs to ASCs, other types of cells, including differentiated cells, or ECM, growth factor, oxygen tension, cytokines, pH, and ion concentration. At first, stem cells such as ASCs have ECM that interacts with ASCs directly.
In general, stem cells maintain their stemness that is supported by their ECM such as glycosaminoglycans (GAGs), which consist of repeating disaccharide units. The GAGs protect stem cells from depletion and over proliferation. There are several types of GAGs such as chondroitin sulfate, dermatan sulfate, keratan sulfate, heparins and heparan sulfates, as well as hyaluronan. Those types of GAGs have linear negatively charged macromolecules and contacted to positively charge of amino acid such as lysine and arginine residues to form GAG-protein complexes (proteoglycans) except hyaluronan, which is lack of a sulfate group (11).
The hyaluronan that is secreted directly into ECM by cell did not covalently bind proteins to from proteoglycans. In addition, the GAGs might interact with protein through hydrogen bonding, van der Waals forces and hydrophobic forces. A lot of interaction between GAGs and protein protected the GAGs from degradation and conformational changes. It was reported that those variations of GAGs have different biological activities that depend on their properties such as molecular weight, as well as bonds between disaccharide. However, the GAGs surrounding stem cells consist of low-sulfated GAGs, which keep the stem cells to interact with many types of growth factors and promote receptor binding. It helps to protect the stem cells to keep their undifferentiated state (12).
Creating a specific microenvironment is the way of GAGs to maintain the undifferentiated state of stem cells, which act as a biochemical and physical barrier. The GAGs might be a selective barrier to molecules that diffuse freely based on their size such as Ca2+ and Na+ ions. Those GAGs interaction with stem cells also initiate homeostasis of extracellular cations (12). Lack of growth factors or receptor binding can have an impact on the stem cells by directly suppressing the over activity of proliferation or differentiation. Furthermore, when the stem cells are promoted to proliferate and differentiate, the cell divides into two cells. The daughter cell translocates to the outside niche of GAGs. It initiates the daughter cell to be exposed to proteins around the cells and activates the signaling pathway (13). The process of proliferation or differentiation that might occur to a cell depends on the signal to the cell. Differentiated stem cell induces the cell to loss its pluripotent ability that is accompanied by the changing of GAGs and proteoglycans patterns in the ECM around the cell.
Adipogenesis occurs in a suitable adipogenic niches where PPARɣ ligands play a critical role to control ASCs niche (8). Once the ASCs differentiated into adipocyte, the cells regulated to synthesis the suitable ECM. Although adipocyte produces the ECM, the type of ECM might be different between different sources of adipose tissue. In normal condition, the adipocytes that are isolated from subcutaneous WAT (SAT) secrete ECM that consists of type I, III and V collagen, while adipocytes isolated from visceral white adipose tissue (VAT) consists of type VI (α1, α2, α3) collagen. Furthermore, in normal state, adipocytes that are isolated from SAT, have a relatively small size, and SAT contains the highest number of adipocyte and secretes leptin. In contrast, adipocytes isolated from VAT have a higher size than SAT’s adipocytes and secrete adiponectin, fibronectin 1 and laminin (5). Therefore, different source of adipose tissue has different microenvironment. In addition, in normal condition, the niche of adipose tissue supports angiogenesis through vascular endothelial growth factor (VEGF) and platelet-derived growth factor (PDGF) as regulatory signals (7,14).
Endothelial cells, pericytes as well as stem or progenitor cells contribute to vessel remodeling to enhance angiogenesis (15). However, adipose tissue not only has angiogenesis activators but also inhibitors (16). The pro-angiogenic factors involve VEGF, acidic fibroblast growth factor (aFGF), insulin like growth factor binding protein-3 (IGFBP-3), monocyte chemoattractant protein-1 (MCP-1), and hepatocyte growth factor (17,18). Antiangiogenic factors involve adiponectin, endostatin, thrombospondin-1, soluble VEGF receptor type 2, and transforming growth factor-beta (TGFb), which are secreted by adipose tissue (16). The secretion of pro-angiogenic and antiangiogenic factors depends on the microenvironment condition. However, in some cases, the alteration of adipose tissue microenvironment may disturb the regulation of angiogenesis that leads to uncontrolled processes. Alteration of microenvironment also affects ASC properties that are caused by deregulated metabolism in adipose tissue (19). On the other hand, due to microenvironment alteration, the function of niche on protection of cell might be reduced which leads to metabolic disorder (2).
Microenvironment of ASCs in T2DM
Numerous studies showed that adipocytes in metabolic disorder such as T2DM are triggered to adipocyte expansion that leads to hypertrophy. In T2DM, the hypertrophic adipocyte accumulation increases TG storage. Accumulation of hypertrophic adipocytes causes excess of nutrient consumption, which induces initial weight (obesity). Moreover the adipocytes contain a greater quantity of mitochondria, therefore produce more reactive oxygen species (ROS) and reduce the sensitivity to insulin as well as increase the blood glucose. The cells also express proinflammatory cytokines that are mediated by hypoxia-inducible factor-1 alpha (HIF-1α). The HIF-1α increases the interleukin 6 (IL-6) and macrophage inflammation factor 1 (MIF-1) (20). Based on the location of adipose tissue, VAT has a higher proinflammatory response than SAT in human body, including IL-6, monocyte attractant protein 1, tumor necrosis factor α (TNF-α) that might contribute to the metabolic effect (21). However, the inflammation mediated immune cell infiltration to the tissue and remodeling of the ECM, as well as vascularization may occur (22,23). Once the adipocyte becomes hypertrophy, inflammation and higher level of ROS occurs, followed by immune cell infiltration to reconstruct the ECM in T2DM.
The hypertrophic adipocytes from SAT express type I collagen α2 and metalloproteinase inhibitor 1, while the hypertrophic adipocytes from VAT express type IV collagen α2, and type VI collagen α1, α2, α3. In the hypertrophic state, the adipocytes from VAT are still higher size than adipocytes in SAT (5). In addition, the hypertrophic adipocytes from SAT and VAT also express highest level of pro-inflammatory factors, and decreased adiponectin that initiates the elevation of M1 macrophages infiltration. However, the formation of ECM and cytokine or chemokine around the adipose tissue is followed by increasing the size of adipocytes (hypertrophy) that trigger insufficiency of oxygen or hypoxia (2).
Hypoxia increases the chance of tissue dysfunction by reducing the expression of genes that are related to angiogenesis such as VEGF, stromal-derived factor-1 (SDF-1) and basic fibroblast growth factor (bFGF). In normal condition of hypoxia, the tissue stabilizes the hypoxia by increasing the expression of transcription factors that regulate oxygen homeostasis (VEGF, bFGF, SDF-1). However, the expression of genes that are related to oxygen homeostasis becomes reduced in T2DM due to overproduction of superoxide anion, followed by the angiogenic dysfunction (24). In contrast, cytokines and chemokines such as tumor necrosis factor alpha (TNF-α) and interleukin-1 beta (IL-1β) are produced by adipose tissue macrophages (ATMs) in an M1 pro-inflammatory state, and lead to the development of insulin resistance (2,25). Moreover, highest level of TNF-α disturbs insulin signaling in adipocytes, while those chemokine recruits the immune cells (leukocytes) to invade into adipose tissue (2,25). Those mechanisms lead the necrosis and apoptosis of cells in T2DM. The necrotic cell death might be cleared by macrophages and causes secretion of several chemokines, while apoptotic cells occur due to increasing expression of apoptotic related gene (26).
Previous studies reported that the expression of apoptotic related genes, i.e. p53, p21, and BAX increased, while Bcl-2 expression that was known as anti-apoptotic gene was confirmed to be expressed as well. However, the result seemed to favor apoptosis process. Decreasing of mRNA expression of Nanog, sox2, oct4 in T2DM was also reported, which indicated the decreasing pluripotency ability (26). Moreover, those effects may shift the role of niche towards ASCs differentiation into adipocytes, which induce the expansion adipocyte number (6,24,25). It was suggested that in T2DM, the microenvironment has the highest level of oxidative stress, blood glucose as well as hypoxia, which may disrupt the ASCs niche especially the ECM component such as GAGs which leads the ASCs to be exposed to biological agents directly to promote proliferation or differentiation.
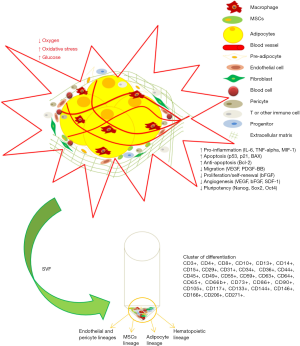
Figure 1 shows adipose tissue microenvironment in T2DM and the final product of SVF.
SVF
In 2001, the SVF that was isolated from human adipose tissue was reported as another type of adult stem cells, which can be differentiated into adipocyte, chondrocyte and osteocyte (27). SVF contains a lot of cells in a variety of frequency, which mainly consists of blood-derived cells, followed by endothelial cells and stromal or progenitor stem cells (28,29). SVF is easily isolated from fat tissue using liposuction technique, which is less invasive compared to bone marrow puncture to get bone marrow MSCs, poses no ethical concerns, as well as has the same differentiation ability as bone marrow derived MSCs that were previously isolated (30). However, the purity and consistency, as well as differentiation ability of SVF depends on the isolation method such as enzymatic or mechanical methods and the source site of adipose tissue such as subcutaneous, omentum or other sources (30-32).
To isolate SVF from adipose tissue, enzymatic method is widely used, which digests collagen fibres around the fat tissue, to separate the cells in the infranatant from floating phases. The pellet phase, which derived from centrifugation of the infranatant, is the SVF, while the floating phase mainly contains digested adipose tissue and lipids. To enhance separation of SVF and floating phase, centrifugation can be used as an alternative method. In addition, filtration helps to capture only cells based on cell size, while washing reduces enzymatic residue (14,31). Although enzymatic method reduces processing time and cost, this method requires infrastructure, expertise and consumables (14).
Several studies reported that SVF could be isolated by a closed system automated biomedical devices, which used enzymatic digestion, gravity separation, filtration or mechanical methods (14,33). Those devices were developed with the aim to make the procedure as easy as possible to be used by non-expert, and to cut the cost of infrastructure, consumables and expertise. Lipogems is an example of a closed system device, which processed lipoaspirates using mild mechanical forces. This device does not require enzyme or additive such as collagenase to digest lipoaspirate (33). However, the use of biomedical devices should be suitable to the user needs, so that comparison studies between the devices are needed to be done first. In order to enhance the outcomes of SVF, an important thing is not only choosing the methods of SVF isolation, but also the fat sources should be noticed.
Festy et al. (32) tried to determine the best SVF contents in different sources between subcutaneous and omental adipose tissue using multicolour flowcytometry. However, the result showed no significant difference in surface marker of CD10, CD13, CD34, CD36, CD55, CD59, and CD65 (32). In contrast, another study reported the stemness features of cells in SVF, which was isolated from superficial adipose tissue (SAT) and deep layer adipose tissue (DAT). Overall, SAT showed increased stem cell progenitors with a higher stromal compound and increased multi-potency of the cells (34). Furthermore, the use of SVF as alternative metabolic disorder disease therapy is rising globally because of its contents and potentials. As SVF therapy number increases, identification of SVF markers is needed to help user to make a decision which marker will be used to characterize SVF.
Following MSC specific surface marker characterization, the markers for SVF started to be reported. Since the SVF is a heterogeneous cell population, the criteria for characterizing SVF cellular contents are determined by surface antigens using multicolour flowcytometry, which showed 31 types of cluster of differentiation (CD) molecules (Table 1) (7,28-30,32-42).
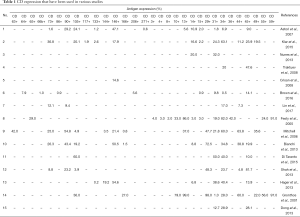
Full table
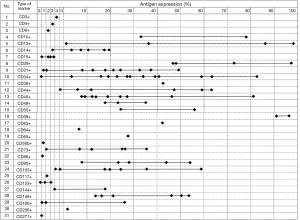
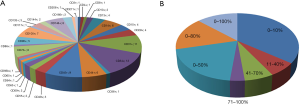
The various CD showed varieties of lineage types, which could be classified into adipocyte, hematopoietic, endothelial, pericytes and MSCs lineages. The highest antigen expression was CD13 (99.0%) (42). However, the lowest antigen marker was CD133 (0.2%) (29). In addition, the highest number of markers (thirteen specific markers) was used and reported by two studies on SVF. Although there are thirteen specific markers that were used, only three similar markers were reported by both studies, which were CD13, CD31, and CD34. A significant difference in the results of both was on CD13 marker. One of the study reported CD13 expression of 86.0%, while the other reported 37.0% (32,40). The difference in values of antigen expression of common markers, which were used in various studies, is showed in Figure 2.
CD34 was the most used marker to characterize the SVF, which was used by 13 studies, followed by CD31 that was used by 10 studies, CD45 (7 studies), CD14, CD44, CD73, CD146 (6 studies) and CD90 (5 studies). The rest of markers were reported by less than 5 studies with the majority were used by one study only. Those common markers, which were reported by more than 4 studies, lead to a specific lineage that is endothelial, hematopoietic and MSCs. However, there are a wide variety of results in antigen expression such as CD34 as the most common marker that has been used (Figure 3). The variety of results of CD34 ranged from 0 to 100%, similar with CD13, CD29 and CD45, and then followed by CD44 and CD146, which were expressed between 0 to 80%. In addition, CD31 (the second common marker that was used), along with CD14, CD73, and CD90 showed less variety i.e., between 0 to 50%. Overall, MSC marker in SVF except CD44 (0 to 80%), such as CD73, CD90, CD105, CD49, CD117, CD133, and CD271 were consistently reported to be less than 51%. However, hematopoietic (both of lymphoid and myeloid lineages), endothelial and pericyte lineages had a wide range of expression, which were 0 to 100%, while adipocyte lineage showed a range of 40 to 70%. Interestingly, there were some markers that showed a more narrow gap of antigen expression.
The markers of CD3, CD4, CD8, CD15, CD64, CD66b, CD86, CD117, CD133, CD206, and CD271 was reported to be expressed in a range of 0 to 10%, followed by CD49, and CD65, which was expressed between 11% and 40%. However, the markers of CD36 and CD73 were expressed in a range of 41% to 70%, while the only CD59 marker was reported between 71% and 100%. The marker of CD59 was consistently reported in high expression. Based on the data, SVF contains many types of cells, which can be classified into several groups i.e., adipocyte, hematopoietic (lymphoid and myeloid), endothelial, pericytes, mesenchymal cell lineage.
Conclusions
The microenvironment changes due to T2DM were due to increase in blood glucose levels and oxidative stress as well as decrease in oxygen level. Those conditions increase the risk factor of inflammation and apoptosis, decrease proliferation or self-renewal, migration, angiogenesis and pluripotency, as well as remodelling of ECM, which alter the microenvironment (niche) of cell. In addition, adipose-derived SVF that is isolated from T2DM patient consists of many types of cells including stem cells, pre-adipocyte, hematopoietic, endothelial, pericytes and immune cells.
Acknowledgments
None
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Tabish SA. Is diabetes becoming the biggest epidemic of the twenty-first century? Int J Health Sci (Qassim) 2007;1:V-VIII. [PubMed]
- Krüger K. Inflammation during obesity–pathophysiological concepts and effects of physical activity. Inflammation 2017;68:163-8.
- Mazini L, Rochette L, Amine M. Regenerative Capacity of Adipose Derived Stem Cells (ADSCs), Comparison with Mesenchymal Stem Cells (MSCs). Int J Mol Sci 2019;20:2523. [Crossref] [PubMed]
- Donnelly H, Salmeron-Sanchez M, Dalby MJ. Designing stem cell niches for differentiation and self-renewal. J R Soc Interface 2018;15:1-18. [Crossref] [PubMed]
- Schoettl T, Fischer IP, Ussar S. Heterogeneity of adipose tissue in development and metabolic function. J Exp Biol 2018;221:jeb162958. [Crossref] [PubMed]
- Hildebrand S, Stümer J, Pfeifer A. PVAT and its relation to brown, beige, and white adipose tissue in development and function. Front Physiol 2018;9:70. [Crossref] [PubMed]
- Klar AS, Guven S, Zimoch J, et al. Characterization of vasculogenic potential of human adipose-derived endothelial cells in a three-dimensional vascularized skin substitute. Pediatr Surg Int 2016;32:17-27. [Crossref] [PubMed]
- Jiang Y, Berry DC, Jo A, et al. A PPARγ transcriptional cascade directs adipose progenitor cell-niche interaction and niche expansion. Nat Commun 2017;8:1-16. [Crossref] [PubMed]
- Berry R, Rodeheffer MS, Rosen CJ, et al. Adipose Tissue-Residing Progenitors (Adipocyte Lineage Progenitors and Adipose-Derived Stem Cells (ADSC)). Curr Mol Biol Rep 2015;1:101-9. [Crossref] [PubMed]
- Luo L, Liu M. Adipose tissue in control of metabolism. J Endocrinol 2016;231:R77-99. [Crossref] [PubMed]
- Soares da Costa D, Reis RL, Pashkuleva I. Sulfation of glycosaminoglycans and its implications in human health and disorders. Annu Rev Biomed Eng 2017;19:1-26. [Crossref] [PubMed]
- Smith RAA, Meade K, Pickford CE, et al. Glycosaminoglycans as regulators of stem cell differentiation. Biochem Soc Trans 2011;39:383-7. [Crossref] [PubMed]
- Gasimli L, Hickey AM, Yang B, et al. Changes in glycosaminoglycan structure on differentiation of human embryonic stem cells towards mesoderm and endoderm lineages. Biochim Biophys Acta 2014;1840:1993-2003. [Crossref] [PubMed]
- Bora P, Majumdar AS. Adipose tissue-derived stromal vascular fraction in regenerative medicine: a brief review on biology and translation. Stem Cell Res Ther 2017;8:145-54. [Crossref] [PubMed]
- Han S, Sun HM, Hwang KC, et al. Adipose-derived stromal vascular fraction cells: update on clinical utility and efficacy. Crit Rev Eukaryot Gene Expr 2015;25:145-52. [Crossref] [PubMed]
- Cao Y. Angiogenesis modulates adipogenesis and obesity. J Clin Invest 2007;117:2362-8. [Crossref] [PubMed]
- Rittig K, Dolderer JH, Balletshofer B, et al. The secretion pattern of perivascular fat cells is different from that of subcutaneous and visceral fat cells. Diabetologia 2012;55:1514-25. [Crossref] [PubMed]
- Horimatsu T, Kim HW, Weintraub NL, et al. The role of perivascular adipose tissue in non-atherosclerotic vascular disease. Front Physiol 2017;8:969. [Crossref] [PubMed]
- Panina YA, Yakimov AS, Komleva YK, et al. Plasticity of adipose tissue-derived stem cells and regulation of angiogenesis. Front Physiol 2018;9:1656. [Crossref] [PubMed]
- Sun K, Tordjman J, Clément K, Scherer PE. Fibrosis and adipose tissue dysfunction. Cell Metab 2013;18:470-7. [Crossref] [PubMed]
- Kwok KHM, Lam KSL, Xu A. Heterogeneity of white adipose tissue: molecular basis and clinical implications. Exp Mol Med 2016;48:e215. [Crossref] [PubMed]
- Sun K, Kusminski CM, Scherer PE. Adipose tissue remodeling and obesity. J Clin Invest 2011;121:2094-101. [Crossref] [PubMed]
- Wernstedt Asterholm I, Tao C, Morley TS, et al. Adipocyte inflammation is essential for healthy adipose tissue expansion and remodeling. Cell Metab 2014;20:103-18. [Crossref] [PubMed]
- Rodrigues M, Wong VW, Rennert RC, et al. Progenitor cell dysfunctions underlie some diabetic complications. Am J Pathol 2015;185:2607-18. [Crossref] [PubMed]
- Kornicka K, Houston J, Marycz K. Dysfunction of mesenchymal stem cells isolated from metabolic syndrome and type 2 diabetic patients as result of oxidative stress and autophagy may limit their potential therapeutic use. Stem Cell Rev Rep 2018;14:337-45. [Crossref] [PubMed]
- Pérez LM, Bernal A, de Lucas B, et al. Altered metabolic and stemness capacity of adipose tissue-derived stem cells from obese mouse and human. PLoS One 2015;10:e0123397. [Crossref] [PubMed]
- Zuk PA, Zhu MIN, Mizuno H, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Engineering 2001;7:211-28. [Crossref] [PubMed]
- Dong Z, Peng Z, Chang Q, Lu F. The survival condition and immunoregulatory function of adipose stromal vascular fraction (SVF) in the early stage of nonvascularized adipose transplantation. PLoS One 2013;8:e80364. [Crossref] [PubMed]
- Hager G, Holnthoner W, Wolbank S, et al. Three specific antigens to isolate endothelial progenitor cells from human liposuction material. Cytotherapy 2013;15:1426-35. [Crossref] [PubMed]
- Lin HR, Heish CW, Liu CH, et al. Purification and differentiation of human adipose-derived stem cells by membrane filtration and membrane migration methods. Sci Rep 2017;7:40069. [Crossref] [PubMed]
- Oberbauer E, Steffenhagen C, Wurzer C, et al. Enzymatic and non-enzymatic isolation systems for adipose tissue-derived cells: current state of the art. Cell Regeneration 2015;4:7. [Crossref] [PubMed]
- Festy F, Hoareau L, Bes-Houtmann S, et al. Surface protein expression between human adipose tissue-derived stromal cells and mature adipocytes. Histochem Cell Biol 2005;124:113-21. [Crossref] [PubMed]
- Bianchi F, Maioli M, Leonardi E, et al. A new nonenzymatic method and device to obtain a fat tissue derivative highly enriched in pericyte-like elements by mild mechanical forces from human lipoaspirates. Cell Transplant 2013;22:2063-77. [Crossref] [PubMed]
- Di Taranto G, Cicione C, Visconti G, et al. Qualitative and quantitative differences of adipose-derived stromal cells from superficial and deep subcutaneous lipoaspirates: a matter of fat. Cytotherapy 2015;17:1076-89. [Crossref] [PubMed]
- Astori G, Vignati F, Bardelli S, et al. "In vitro" and multicolor phenotypic characterization of cell subpopulations identified in fresh human adipose tissue stromal vascular fraction and in the derived mesenchymal stem cells. J Transl Med 2007;5:55-64. [Crossref] [PubMed]
- Nunes SS, Maijub JG, Krishnan L, et al. Generation of a functional liver tissue mimic using adipose stromal vascular fraction cell-derived vasculatures. Sci Rep 2013;3:2141. [Crossref] [PubMed]
- Traktuev DO, Merfeld-Clauss S, Li J, et al. A population of multipotent CD34-positive adipose stromal cells share pericyte and mesenchymal surface markers, reside in a periendothelial location, and stabilize endothelial networks. Circ Res 2008;102:77-85. [Crossref] [PubMed]
- Crisan M, Yap S, Casteilla L, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 2008;3:301-13. [Crossref] [PubMed]
- Brown JC, Shang H, Li Y, et al. Isolation of adipose-derived stromal vascular fraction cells using a novel point-of-care device: cell characterization and review of the literature. Tissue Eng Part C: Methods 2017;23:125-35. [Crossref] [PubMed]
- Mitchell JB, McIntosh K, Zvonic S, et al. Immunophenotype of human adipose-derived cells: temporal changes in stromal-associated and stem cell-associated markers. Stem Cells 2006;24:376-85. [Crossref] [PubMed]
- Shah FS, Wu X, Dietrich M, et al. A non-enzymatic method for isolating human adipose tissue-derived stromal stem cells. Cytotherapy 2013;15:979-85. [Crossref] [PubMed]
- Gronthos S, Franklin DM, Leddy HA, et al. Surface protein characterization of human adipose tissue-derived stromal cells. J Cell Physiol 2001;189:54-63. [Crossref] [PubMed]
Cite this article as: Karina, Pawitan JA, Rosadi I. Adipose-derived stem cells and their microenvironment (Niche) in type 2 diabetes mellitus. Stem Cell Investig 2020;7:2.