
Complete remission of aggressive T-cell LGL leukemia with pentostatin therapy: first case report
Introduction
Large granular lymphocytic (LGL) leukemia is a rare lymphoproliferative disorder defined by monoclonal proliferation of mature T cells or natural killer cells. In 1993, LGL leukemia was divided into two subtypes: T-cell LGL (T-LGL) leukemia and NK-cell leukemia, later recognized by the World Health Organization in 2001 (1). The T-cell variant is more common, accounting for 85% of reported cases and is typically indolent. This variant is associated with STAT3 mutation and displays a constitutive mature post-thymic phenotype: CD3+/TCRαβ+/CD4–/CD8+/CD57+ (1). The NK-cell variant, typically associated with Epstein-Barr virus, is more aggressive, and patients have a significantly poorer prognosis. There is a separate entity defined as chronic NK-cell LGL lymphocytosis that presents similarly to chronic T-LGL leukemia and shares the same STAT3 genetic mutation hallmark (1). Both NK-cell LGL subtypes share an immunophenotypic profile that includes: CD3ε+/TCRαβ–/CD4–/CD8+/CD16+/CD56+. Several cases of aggressive T-LGL leukemia have been reported in the past two decades, identifying a rare variant of LGL leukemia that displays a CD4+ T-cell phenotype, with or without CD8 co-expression, and often has STAT5B mutations (1-10). Unlike those with the indolent T-cell variant, patients with the aggressive variant present acutely, demonstrating non-specific constitutional symptoms, hepatosplenomegaly, lymphocytosis, and cytopenias (2-10).
Indolent LGL leukemia is commonly treated with immunosuppressive therapy given the composition of constitutively activated T cells (1,11). Complete response, defined as complete normalization of blood counts, and an absence of circulating LGL cells, has been reported with a variety of monotherapies including methotrexate (MTX), cyclophosphamide, and cyclosporine. Purine analogs and monoclonal antibodies have also been used for chronic T-LGL leukemia with varying degrees of success (11).
Since the aggressive form of T-LGL leukemia is rare, there is no standardized consensus on treatment. Previous case reports have described different combinations, including steroids, immunosuppressive agents, and chemotherapy-based regimens similar to those used for acute lymphocytic leukemia (ALL), but most patients’ disease progressed and resulted in fatality (1,11). Thus, there is a need to identify alternate therapies that can effectively treat this aggressive disease. Herein, we present the following case of aggressive T-LGL leukemia refractory to two lines of therapy with achievement of complete remission after treatment with pentostatin in accordance with the CARE reporting checklist (available at http://dx.doi.org/10.21037/sci-2020-035).
Case presentation
A 55-year-old African American male with hypertension, type II diabetes mellitus, hyperlipidemia, and gout presented to a community hospital emergency department (ED) complaining of multiple abdominal ecchymosis, which appeared over the previous 2–3 weeks. He described associated symptoms of fatigue, lower extremity edema, blurry vision, anorexia, and unintentional weight loss over the past month. In the ED, he was noted to have leukocytosis to >100 k/µL, and a preliminary diagnosis of acute leukemia was made. He was transferred to Medstar Georgetown University Hospital for further evaluation. On exam, patient was alert and oriented with abdominal ecchymosis and lower extremity edema. He was noted to have a leukocytosis of 101 k/µL, hemoglobin of 9.7 gm/dL, and platelet count of 54 k/µL with significant hepatosplenomegaly with absence of lymphadenopathy (LAD). His initial liver function tests (LFTs) were alanine aminotransferase (ALT) 18 µ/L, aspartate aminotransferase (AST) 178 µ/L, alkaline phosphatase 98 µ/L, total bilirubin 1.0 mg/dL, albumin level 3.2 gm/dL, and lactate dehydrogenase (LDH) of 8,082 units/L. Hydroxyurea was initiated while awaiting results of diagnostic testing.
Peripheral blood flow cytometry confirmed a diagnosis of T-LGL leukemia with 80% atypical cells CD2+/CD3+/CD4+/CD8+/CD56–/HLA-DR+/CD16+ (37%)/CD11b+ (37%)/CD5+/CD7–/TdT–/CD34– profile. Bone marrow biopsy revealed a normocellular marrow with trilineage hematopoiesis including adequate megakaryocytes. However, aspirate flow cytometry showed mature T-cell lymphoid leukemia with LGL features and blastic cells, consistent with a diagnosis of the aggressive form of T-LGL leukemia. PCR analysis of aspirate was positive for clonal TCRβ rearrangement.
Based on his clinical presentation, he was started on hyper-CVAD (cyclophosphamide, vincristine, doxorubicin, and dexamethasone) cycle A1 with levofloxacin, acyclovir, and fluconazole prophylaxis. A lumbar puncture to assess CNS involvement and for intrathecal (IT) MTX CNS prophylaxis was planned, however, the patient began complaining of new daily headaches. A brain MRI revealed a subacute cerebellar vermis hemorrhage, resulting in the postponement of the lumbar puncture (Figure 1). Cycle B1 was complicated by severe headaches and ataxia consistent with cerebellar toxicity likely related to high dose cytarabine, but repeat brain MRI revealed improvement in the hematoma. Systemic intravenous (IV) cytarabine was discontinued due to the adverse neurological effects.
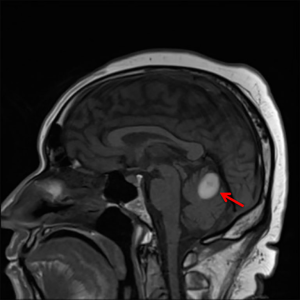
He completed cycle 2A of hyper-CVAD and received a diagnostic lumbar puncture with CSF sampling and IT cytarabine. His CSF flow revealed no evidence of malignancy. On admission for cycle 2B, he was found to have decreased ANC after previously normalizing his cell counts, prompting reassessment of his disease status with peripheral blood flow cytometry. The results revealed persistent T-LGL leukemia, confirmed by bone marrow aspirate flow cytometry. Approximately two-thirds of his lymphoid gate was atypical with CD3+/CD4+/CD8+/CD56–/HLA-DR+ phenotype and decreased CD2, CD5, and CD7.
Due to the refractory nature of his disease, his treatment was changed to alemtuzumab, with standard starting dose of 3 mg and titration up to 30 mg IV three times a week with Bactrim prophylaxis. The patient experienced side effects of fatigue, rigors and chills after infusions, requiring IV meperidine to control his symptoms. No improvement on flow cytometry or clinically was seen after 10 doses of alemtuzumab and pentostatin 4 mg/m2 IV weekly for four doses followed by pentostatin 4 mg/m2 IV every 2 weeks was added to his regimen (12). His LFTs at the start of pentostatin treatment were ALT 40 µ/L, AST 140 µ/L and alkaline phosphatase 257 µ/L. After two doses of pentostatin, the patient experienced pancytopenia with an absolute neutrophil count (ANC) of 200, hemoglobin 7.7 gm/dL and platelets 10 k/µL requiring transfusions, and both drugs were held. Two weeks later, single agent pentostatin was restarted. One month after treatment, bone marrow was repeated, and patient was found to be in complete remission with flow cytometry analysis negative for residual T-LGL cells. Over the subsequent months, the patient reported increased energy and appetite, with improved LFTs to ALT 14 µ/L, AST 20 µ/L, alkaline phosphatase 94 µ/L and complete blood count values of ANC 1.8 k, hemoglobin 9.6 gm/dL, and platelets 203 k/µL. Patient received a total of 14 doses of pentostatin, which was the maximum studied (12), however, the patient needed to continue therapy while waiting for his peripheral blood stem cell (PBSC) transplant from his son. The patient received his bone marrow transplant (BMT) on September 13, 2019. He required G-CSF support but ultimately achieved hematological recovery with an ANC >500/mcL 2 weeks after his transplant. His transplant was complicated by acute skin graft versus host disease treated with prednisone and tapered in November 2019. His 3-month bone marrow biopsy after BMT was normocellular with trilineage hematopoiesis and no evidence of T-LGL with 100% donor chimerism. At the most recent follow up in April 2020, the patient was doing well with no evidence of infection, no rash and only mild ongoing neuropathy in his feet.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images.
Discussion
Currently, there is no consensus on treatment for aggressive T-LGL leukemia. Its atypical presentation and variety in immunophenotypes continue to challenge clinicians and their attempts to standardize diagnostic and therapeutic approaches. Gentile et al. first reported the disease in 1994, and since then, only three reports of complete remission have been described using different treatments as outlined in Table 1 (2-10). Two of these patients’ remissions lasted less than 2 years, and the length of the third remission was not reported (4,5,8). This variability in success requires practitioners to expand the armamentarium of treatment options to include non-traditional agents and make therapy adjustments as needed for their patients with this rare disease. The most common approach to the aggressive T-cell variant is a chemotherapy-based regimen akin to acute leukemia treatment protocols, but as seen in our patient, additional therapies are often required with varying degrees of success.
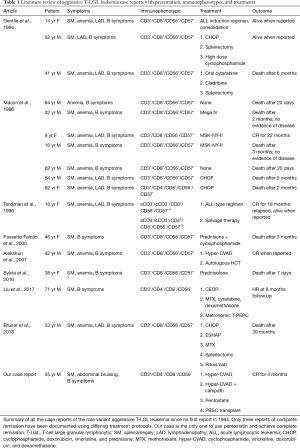
Full table
We report a patient with a T-cell immunophenotype profile of CD3+/CD4–/CD8+/CD56– that presented symptomatically similar to past cases. Once the diagnosis of aggressive T-LGL leukemia with lymphoblastic features was confirmed, hyper-CVAD was chosen as his initial therapeutic regimen due to its demonstrated efficacy in treating ALL (13). Though his IT chemotherapy had to be postponed after discovering his cerebellar vermis hematoma, he eventually received IT cytarabine, and his CSF was negative for malignancy. Unfortunately, our patient did not respond to this treatment protocol, requiring a different approach to combat his persistent disease.
We selected alemtuzumab as his next treatment, based upon its success on T-cell neoplasms reported by Ravandi et al. in 2009 (12). Alemtuzumab alone has been investigated as a monotherapy for classic T-LGL refractory to first-line immunosuppressive therapy in a single arm prospective clinical trial. Benchmark results at 3 months follow up demonstrated a 56% hematologic response but given the efficacy of alemtuzumab in chronic lymphocytic leukemia (CLL), its positive effect on the classic T-LGL leukemia, an indolent disease, is not surprising (14,15). The monoclonal antibody has been studied as monotherapy in more aggressive T-cell diseases, such as HTLV-1-associated adult T-cell leukemia, but the results support use of alemtuzumab with other agents as opposed to use as a single agent (15).
Ravandi et al. treated 24 patients with different T-cell leukemia and lymphomas with alemtuzumab 30 mg IV three times a week for up to 3 months in addition to pentostatin 4 mg/m2 IV weekly for 4 weeks then every other week for up to 6 months. The study reported a 54% response rate with a median response time of 19.5 months. Eleven patients achieved CR, defined as undetectable evidence of disease by peripheral blood, bone marrow morphology and CT scan. Notably, only one of the two patients with T-LGL had partial response, but the variant seen in our patient parallels more aggressive T-cell malignancies such as T-cell prolymphocytic leukemia (T-PLL), which had a 62% CR rate and 69% overall response rate with alemtuzumab and pentostatin (12).
Pentostatin was added to the patient’s regimen as his disease persisted with 10 doses of alemtuzumab monotherapy. However, he consequently experienced severe pancytopenia, leading to a brief drug holiday. Two weeks later, pentostatin alone was restarted since alemtuzumab was ineffective and not tolerated well. Pentostatin has been proven as an effective chemotherapeutic agent in patients with T-PLL and given the significant cytopenias as well as his infusion reactions associated with alemtuzumab, the decision was made to only continue pentostatin (16). After 5 doses of pentostatin, the patient achieved complete remission, but required extended dosing with a total of 18 doses as bridging therapy due to a delay in his PBSC transplant (12).
This is the first reported case of the treatment of aggressive T-LGL leukemia with pentostatin resulting in complete remission. Its success with good tolerability and lack of major complications provides further evidence that pentostatin is a suitable option for patients with aggressive T-cell neoplasms refractory to initial chemotherapy regimens. As a purine analogue and a strong adenosine deaminase inhibitor, pentostatin has been widely used as an anti-lymphocyte treatment, particularly in patients with T-PLL and hairy cell leukemia (17,18). There has been greater research recently, however, to expand indications for pentostatin, including cutaneous T-cell lymphomas and marginal zone lymphoma, with similar reports of high tolerability by patients (19,20).
Our patient did not tolerate his first two rounds of chemotherapy well, experiencing adverse effects that include cerebellar toxicity and infusion reactions thus requiring individualized adjustment of his treatment. On pentostatin monotherapy his fatigue and appetite improved without any additional side effects. Given the inefficacy of his treatment with hyper-CVAD and the superior side effect profile of pentostatin, we believe pentostatin could be considered as an initial therapeutic option, thus reducing the number of toxic agents administered to patients. This case warrants further exploration into pentostatin as a treatment for T-LGL leukemia as well as other T-cell neoplasms. Additionally, further investigation into the similarities between aggressive T-LGL and other T-cell neoplasms that are effectively treated by pentostatin, such as T-PLL and hairy cell leukemia, could potentially provide more data on the pathogenesis of the rare disease.
Conclusions
LGL leukemia is a rare lymphoproliferative disorder currently classified by T-cell or NK-cell proliferation. While the T-LGL variant is classically an indolent disease, there are cases of an aggressive T-LGL leukemia that present with nonspecific B symptoms, SM, cytopenias and progress rapidly. Given the rarity of this form, there lacks a consensus in treatment for these patients. The first therapy is often a high-dose regimen of chemotherapy such as CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) or hyper-CVAD, but its efficacy has remained low, requiring additional agents. Our patient demonstrated improvement in both disease progression and side effect profile once he was started on pentostatin monotherapy after two rounds of hyper-CVAD and 10 doses of alemtuzumab. This case suggests pentostatin is an effective option for refractory disease and could be considered as an initial, less cytotoxic therapy for patients with aggressive T-LGL leukemia and warrants further research into its use for T-cell neoplasms.
Acknowledgments
This work was supported by the Lombardi Cancer Center.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at http://dx.doi.org/10.21037/sci-2020-035
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/sci-2020-035). Dr. CB reports other from Alexion, other from Sanofi, other from Argenx, other from Apellis, outside the submitted work. Dr. CL reports other from Astellas, other from Jazz Pharma, other from Abbvie, other from Macrogenics, other from Agios, other from Daiichi, outside the submitted work. The other author has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lamy T, Moignet A, Loughran TP Jr. LGL leukemia: from pathogenesis to treatment. Blood 2017;129:1082-94. [Crossref] [PubMed]
- Brunet V, Pavic M, Fleury I, et al. Case report of aggressive t-cell large granular lymphocytic leukemia. Blood 2017;130:5189.
- Gentile TC, Uner AH, Hutchison RE, et al. CD3+, CD56+ aggressive variant of large granular lymphocyte leukemia. Blood 1994;84:2315-21. [Crossref] [PubMed]
- Macon WR, Williams ME, Greer JP, et al. Natural killer-like T-cell lymphomas: aggressive lymphomas of T-large granular lymphocytes. Blood 1996;87:1474-83. [Crossref] [PubMed]
- Tordjman R, Macintyre E, Emile JF, et al. Aggressive acute CD3+, CD56- T cell large granular lymphocyte leukemia with two stages of maturation arrest. Leukemia 1996;10:1514-9. [PubMed]
- Passetto Falcão R, Pinto Simões B, Garcia AB, et al. Aggressive variant of morphologically typical T large granular lymphocyte leukemia/lymphoma lacking NK cell markers. Acta Haematol 2000;104:110-4. [Crossref] [PubMed]
- Sylvia MT, Jacob SE, Basu D, et al. CD56 negative aggressive T cell large granular lymphocytic leukemia. Indian J Hematol Blood Transfus 2016;32:121-4. [Crossref] [PubMed]
- Alekshun TJ, Tao J, Sokol L. Aggressive T-cell large granular lymphocyte leukemia: a case report and review of the literature. Am J Hematol 2007;82:481-5. [Crossref] [PubMed]
- Brunet V, Pavic M, Marouan S, et al. Literature review of all cases of aggressive T-cell large granular lymphocytic leukemia cases and report of an additional case. Blood 2018;132:5421. [Crossref]
- Liu Y, Fan L, Zhao H, et al. Metronomic regimen as an effective treatment for aggressive T-LGL leukemia with central nervous system infiltration: clinical experience and review of literature. Oncotarget 2017;8:32292-7. [Crossref] [PubMed]
- Lamy T, Loughran TP Jr. How I treat LGL leukemia. Blood 2011;117:2764-74. [Crossref] [PubMed]
- Ravandi F, Aribi A, O'Brien S, et al. Phase II study of alemtuzumab in combination with pentostatin in patients with T-cell neoplasms. J Clin Oncol 2009;27:5425-30. [Crossref] [PubMed]
- Moskoff B, Chang A, Ho G, et al. Efficacy and tolerability of hyper-CVAD in adult acute lymphoblastic patients: a retrospective analysis at a comprehensive cancer center. Blood 2016;128:5195. [Crossref]
- Dumitriu B, Ito S, Feng X, et al. Alemtuzumab in T-cell large granular lymphocytic leukaemia: interim results from a single-arm, open-label, phase 2 study. Lancet Haematol 2016;3:e22-9. [Crossref] [PubMed]
- Sharma K, Janik JE, O'Mahony D, et al. Phase II study of alemtuzumab (CAMPATH-1) in patients with HTLV-1-associated adult t-cell leukemia/lymphoma. Clin Cancer Res 2017;23:35-42. [Crossref] [PubMed]
- Mercieca J, Matutes E, Dearden C, et al. The role of pentostatin in the treatment of T-cell malignancies: analysis of response rate in 145 patients according to disease subtype. J Clin Oncol 1994;12:2588-93. [Crossref] [PubMed]
- Bagheri S, Saboury AA, Haertlé T. Adenosine deaminase inhibition. Int J Biol Macromol 2019;141:1246-57. [Crossref] [PubMed]
- Andrasiak I, Rybka J, Wrobel T. Response to the therapy in hairy cell leukemia: systematic review and meta-analysis. Clin Lymphoma Myeloma Leuk 2018;18:392-9.e3. [Crossref] [PubMed]
- Zinzani PL, Bonthapally V, Huebner D, et al. Panoptic clinical review of the current and future treatment of relapsed/refractory T-cell lymphomas: cutaneous T-cell lymphomas. Crit Rev Oncol Hematol 2016;99:228-40. [Crossref] [PubMed]
- Khashab T, Hagemeister F, Romaguera JE, et al. Long-term overall- and progression-free survival after pentostatin, cyclophosphamide and rituximab therapy for indolent non-Hodgkin lymphoma. Br J Haematol 2019;185:670-8. [Crossref] [PubMed]
Cite this article as: Krackeler ML, Broome C, Lai C. Complete remission of aggressive T-cell LGL leukemia with pentostatin therapy: first case report. Stem Cell Investig 2020;7:24.


