
Vitamin E and selenium improve mesenchymal stem cell conditioned media immunomodulatory effects
Introduction
Mesenchymal stem cells (MSCs) are multipotent and have the ability to self-renewing and differentiating into different lineages. These cells can be isolated from many tissues such as bone marrow, adipose tissue, and placenta (1). MSCs show immunoregulatory properties and affect the immune system. MSCs prevent differentiation of monocytes into dendritic cells (DCs). They also have inhibitory effects on T and B lymphocytes (2).
Vitamin E (Vit E) is a term for a group of fat-soluble compounds that have divided into tocopherol and tocotrienol. Vit E has antioxidant properties and protects cell membranes against the oxidation-reduction (redox) reactions (3). In different studies have been reported Vit E can increase antibody production and resistant to viral diseases in the elderly (4).
Selenium (Se) is an essential micronutrient that can affect immune response. The effects of Se mainly due to participation in selenoproteins (5). In patients with HIV1, Se deficiency leads to a decrease in T CD4+ frequency and the cytotoxicity effects of the NK cells. Se supplementation also reduces inflammation. Some studies showed relationship between serum Se levels and the risk of autoimmune diseases (6,7).
DCs play an essential role in initiating acquired and innate immunity. However, they could induce both tolerance and activation of the immune response (8). Besides DC there are other cells such as T cell, B cells, NK, and monocyte which are involved in immune responses. As previously stated MSCs with immunoregulatory properties can affect the function of these cells in various ways (2).
Over the past decade, several studies have been performed on the effects of antioxidants such as Vit E and Se on MSCs. However, very few studies have been carried out about the effects of antioxidant on the immunoregulatory characteristics of MSCs. In this study, the effect of MSC conditioned media (CM) treated with Vit E and Se on DC surface markers such as HLA-DR, CD86, CD40, and CD83 and also IL-12, IL-10 and TGF-β levels and T-bet, GATA3, RORγt, and FOXP3 expression levels in peripheral blood mononuclear cells (PBMCs) were studied. We present the following article in accordance with the MDAR reporting checklist (available at http://dx.doi.org/10.21037/sci-2020-008).
Methods
MSC isolation and culture
Adipose tissue was isolated from patients undergoing surgery and washed three times with PBS containing penicillin/streptomycin (Gibco, USA). Adipose tissue was digested with collagenase type IV (Sigma-Aldrich, USA) and incubated for 30 minutes at 37 °C. Cell suspension was passed from 0.7 micron filter and were cultured in DMEM medium (Gibco, USA) containing 10% FBS (Gibco, USA), 1% penicillin/streptomycin and 1% L-Glutamine (Gibco, USA). To examine the ability of differentiation of MSCs to osteocytes 10,000 cells and for adipocytes, 20,000 cells in 1 m/L of DMEM medium with 10% FBS were seeded in four-well plates. After 48 hours, the medium was replaced with a differentiated medium. After 3 weeks, to determine the differentiation of these cells, wells were washed with PBS and stained with Alizarin Red (Sigma-Aldrich, USA (and Oil Red O (Sigma-Aldrich, USA) (Figure S1). Also, MSCs surface markers were investigated using flow cytometry. MSCs were isolated with trypsin and washed with PBS and then, they were suspended in PBS containing 3% BSA. After addition of antibody, expression of CD105, CD90, CD45, CD34 and CD73 (BioLegend, USA) were investigated by flow cytometry (Figure S2) (9,10).
Monocyte-derived DCs
PBMCs were isolated from the whole blood of three healthy individuals by Ficoll (Germany, inno-train). For monocyte isolation 1.5×106 PBMCs were seeded in T25 flask and incubated at 37 °C for 3 hours. After that, suspended cells were removed by washing with PBS and RPMI. Adherence cells were cultured in RPMI medium (Gibco, USA) containing 10% FBS and 1% penicillin/streptomycin plus 20 ng/mL IL-4 (PeproTech, UK) and 50 ng/mL GM-CSF (PeproTech, UK). On day 5, LPS (Sigma, USA) with a concentration of 50 ng/mL and the CM of the MSCs treated with Vit E and Se alone and in combination were added to culture media. On day seven, supernatant and cells were collected for further experiments (Figure S3) (11,12). To determine the maturation of DCs and expression of CD1a, CD83, CD86, CD40, and HLA-DR cells (BioLegend, USA) were isolated with cold PBS-EDTA and washed with staining solution. In each group, the appropriate amount of antibody was added to the cell suspension then, cells were incubated for 45 minutes at 4 °C. After washing with PBS cells were analyzed by flow cytometry (13).
PBMC isolation
PBMCs were isolated from whole blood. A total of 5×105 cells per well were seeded in six-well plates. To each well, 1 mL of complete RPMI medium and 1 mL of the CM of MSCs treated with Vit E and Se was added. The supernatant was replaced and collected every 48 hours. After 7 days, cells were collected for further investigation. They were stored at –70 °C until used.
Cell viability assay
To determine the appropriate and non-toxic concentration of Vit E (alpha-tocopherol, Sigma, USA) and Se (selenomethionine, Sigma, USA), MSCs were seeded in a 96-well plate. After 24 hours, Vit E and Se were added to the medium at different concentrations. 48 hours later, the MTT (Sigma-Aldrich, USA) diluted with DMEM added to wells. After 4 hours in 37 °C Incubator, 100 µL DMSO (Sigma-Aldrich, USA), was added to each well and read at 570 nm wavelengths (14).
Enzyme-linked immunosorbent assay (ELISA)
To detection, the concentration of cytokines, IL-10 (Mabtech, Sweden), IL-12 (Mabtech, Sweden) and TGF-β (Invitrogen, USA) kits were used. The supernatant was collected from mature DCs and PBMCs treated with MSC (Vit E) CM, MSC (Se) CM and MSC (Vit E + Se) CM after 48 hours. Finally, ELISA plates were read by ELISA reader.
Real time-polymerase chain reaction (RT-PCR)
PBMCs were cultured with different groups of MSC CM. After 7 days RNA was isolated from PBMCs with RNA ex plus (Qiagen, Germany). The concentration of RNA was determined by Nanodrop spectrophotometry. Eventually, cDNA (Takara, Japan) was synthesized and Real-time qPCR reactions were carried out using the SYBR Green PCR Master Mix (Biofact, Korea) (Table 1).

Full table
Ethical statement
This study was approved by the ethics board of Iran University of Medical Sciences (IR.IUMS.FMD.REC 1396.9411127004) and informed consent was taken from all individual participants. Furthermore, this work was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Statistical analysis
In the first step, quantitative data were analyzed for normal distribution by the normalization test. The quantitative data presented in this study were non-parametric. For statistical analysis and comparison of the results of the data in the studied groups, Mann-Whitney (between two groups) and Kruskal-Wallis (more than two groups) were used.
Results
Determining the optimal concentration of Vit E and Se by MTT assay
The appropriate concentration of Vit E and Se was determined using MTT assay. According to MTT test results, concentrations of 22 µM for Vit E (P=0.004), 250 µM for Se (P=0.009), and 22 µM Vit E with 300 µM Se combined (P>0.99) were used (Figure S4).
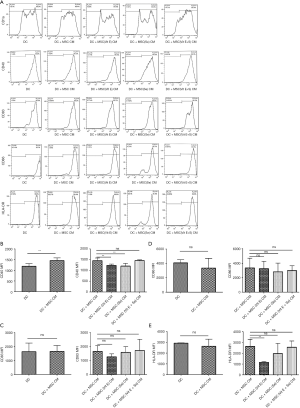
Evaluation of DC maturation treated with MSC (Vit E and Se) CMs
The expression of CD40 in DC + MSC CM was significantly higher compared with DC group (P=0.002). The expression of CD40 in DC + MSC (Vit E) CM and DC + MSC (Se) CM was significantly decreased in comparison to DC + MSC CM (P=0.002). Also, the expression of HLA-DR was reduced only in DC + MSC (Vit E) CM compared with DC + MSC CM (P=0.002). The expression of CD83 and CD86 among the groups were not different (Figure 1).
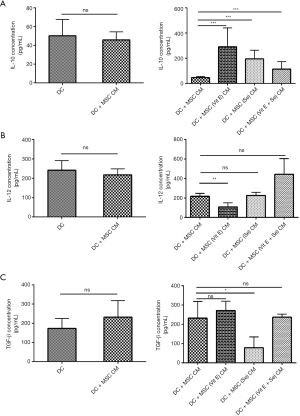
Evaluation of IL-10, IL-12, and TGF-β in the supernatant of myeloid DCs (mDCs) treated with MSC (Vit E and Se) CMs
IL-10 level was significantly increased in DC + MSC (Vit E) CM, DC + MSC (Se) CM and DC + MSC (Vit E + Se) CM compared with DC + MSC CM (P<0.001), although the highest increase was related to DC + MSC (Vit E) CM. In relation to IL-12 level, there was only a significant reduction in DC + MSC (Vit E) CM in comparison to DC + MSC CM (P=0.002) and TGF-β concentration was reduced only in DC + MSC (Se) CM compared with DC + MSC CM (P=0.03) (Figure 2).
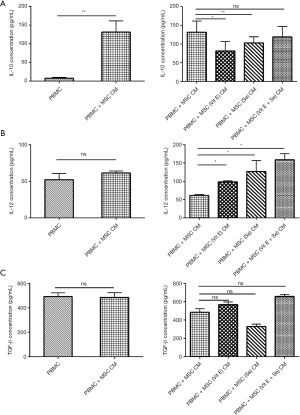
Evaluation IL-10, IL-12, and TGF-β in the supernatant of PBMC treated with MSCs (Vit E and Se) CM
The concentration of IL-10 in PBMC + MSC CM was significantly higher than PBMC (P=0.007). The level of IL-10 in DC + MSC (Vit E) CM (P=0.01) and DC + MSC (Se) CM (P=0.004) in comparison with PBMC + MSC CM was significantly decreased, while the level of IL-12. In DC + MSC (Vit E) CM, DC + MSC (Se) CM and DC + MSC (Vit E + Se) CM has been significantly increased compared with PBMC + MSC CM (P=0.02). Level of TGF-β in DC + MSC (Se) CM was decreased than PBMC + MSC CM, although this was not significant (Figure 3).
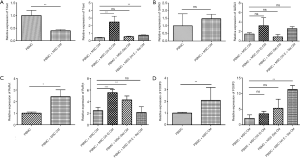
The expression of T-bet, RORγt, GATA3 and FOXP3 in PBMC treated with MSCs (Vit E and Se) CM
The results showed that the expression of T-bet in PBMC + MSC CM was significantly decreased compared with PBMC (P=0.002) while RORγt (P=0.04) and FOXP3 (P=0.004) expression have been significantly increased. The expression levels of T-bet in PBMC + MSC (Vit E) CM and PBMC + MSC (Vit E + Se) CM were increased compared with PBMC + MSC CM (P=0.001) while RORγt expression was increased in PBMC + MSC (Vit E) CM (P=0.04) and PBMC + MSC (Se) CM (P=0.007). FOXP3 expression was increased in PBMC + MSC (Vit E + Se) CM in comparison with PBMC + MSC CM (P=0.004). The expression of GATA3 was not significantly different among groups (Figure 4).
Discussion
According to the obtained results, MSC CM increases the expression of CD40 in mDCs, but there was no significant difference in the expression of CD83, CD86, and HLA-DR. In the same way, the results of Spaggiari and Zhang studies showed that the effect of MSC on DCs is time-dependent. It was also reported that DCs co-cultured with MSCs on the 5th day of differentiation would not reduce CD80, CD83, CD86, and HLA-DR frequency of DCs and also, there was no significant difference in the concentration of IL-10, IL-12 and TGF-β between the two groups. Zhang et al. showed that the levels of IL-10 and IL-12 in DC cultured on the 5th day with LPS and MSC CM, were not significantly different (15,16). van den Berk et al. reported that umbilical cord blood MSCs didn’t prevent DC maturation and increase the expression of CD80 and CD83 and IL-12 level (17). Our results showed that in DC + MSC (Vit E) CM and DC + MSC (Se) CM, expression of CD40 was reduced but, this inhibitory effect was not seen in the DC + MSC (Vit E + Se) CM.
The expression of HLA-DR in the DC + MSC (Vit E) CM showed a significant decrease compared with DC + MSC CM, but in the other groups, there was no difference. In the present study, the level of IL-10 was significantly increased in DC + MSC (Vit E) CM and DC + MSC (Se) CM and DC + MSC (Vit E + Se) CM in comparison to the control group. Also, IL-12 level decreased significantly in the DC + MSC (Vit E) CM compared with the control group. According to the results, it seems that Vit E and Se increasing MSC survival and stability and thus improved the immunomodulatory effect of MSCs on DC (18).
LPS activates NF-ĸB and cause DC maturation. Prevent expression of NF-ĸB inhibit DC maturation (19). Several studies showed that MSC CM in osteoarthritis model increase inhibitory-κ-B-α (IκBα) and inhibit expression of NF-ĸB. Besides, reactive oxygen species (ROS) lead to the activation of NF-ĸB, and many antioxidants, especially Vit E, have the ability to prevent the activation of NF-ĸB through this way and can prevent DC maturation (20,21).
Regarding the results, it seems that the MSC CM treated with Vit E and Se improve the immunoregulatory effects of MSC and they could better decrease DC maturation markers and IL-12 level and increase IL-10 concentration. In addition, there might be free Vit E and Se in MSC CM, in which case the two antioxidants will also prevent DC maturation by inhibiting NF-ĸB.
In the next step of this study, results showed that the levels of IL-10 in PBMC + MSC CM compared with the PBMCs group significantly increased. In this regard, Kim et al. reported that MSCs derived from human adipose tissue show an inhibitory effect on the proliferation of PBMCs (22).
In the PBMCs + MSC (Vit E) CM and PBMCs + MSC (Se) CM, the IL-10 level was significantly reduced compared with the PBMCs treated with MSC CM. IL-12 level was significantly increased in PBMC + MSC (Vit E) CM and PBMC + MSC (Se) CM. The study of Hernández et al. showed that in PBMCs treated with Vit E IL-10 and IL-4 concentration reduce, and the expression of the T-bet increase (23). On the other hand, Wassall et al. showed that Vit E has no specific effect on cytokine responses in people with PBMCs (24).
Daeian et al. found that Se supplementation does not have any effect on the production of proinflammatory cytokines in the peripheral blood of patients undergoing hematopoietic stem cell transplantation (HSCT) (25).
The expression levels of GATA3, FOXP3, RORγt, and T-bet in PBMCs treated with different groups of MSCs CM was studied. T-bet and RORγt expression in the PBMC + MSC (Vit E) CM was significantly increased compared with the PBMC + MSC CM. There was no significant difference in the expression of GATA3 gene between the two groups. The treatment of PBMC + MSC (Se) CM suppressed the expression of RORγt. The expression of FOXP3 increased significantly in PBMC + MSC (Vit E + Se) CM.
Kay et al. reported MSC CM in patients with RA increased the levels of IL-10 and FOXP3 in T cells (26). Agree with our results, Ivanova-Todorova et al. found that the MSCs isolated from the adipose tissue increase the expression of FOXP3 and the concentration of IL-10 (27).
Rozenberg et al. reported MSCs suppress T cells activity via the PD-1 pathway. Luz-Crawford et al. observed that MSCs regulate Th1 and Th17 response. The MSC CM through PGE2 increased the differentiation of Th17 cells (28,29).
Adolfsson et al. reported that Vit E increase the proliferation of T cells in the mouse (30). Besides, Zingg et al. showed that alpha-tocopherol activates many genes in a dose-dependent manner and increase the proliferation and survival of T cells compared to other Vit E isoforms (31).
As previously mentioned, Se increase the survival of stem cells. Limited studies have been evaluated the immunomodulatory effects of Se-treated MSCs. Sang et al. observed that sodium selenite reduce RORγt and T-bet expression levels accompanied with increased frequency of Treg in colitis mice (32). Dustan et al. stated that there is no relationship between IL-10 produce from lymphocyte and Se levels (33). The results are controversial about the effects of Se on immune system responses.
Conclusions
Finally, regarding the results of this study and previous studies, in some cases, such as increasing the expression of RORγt as well as FOXP3 in the group where these two substances were combined, treatment of MSC with Vit E and Se increased immunomodulatory effects of MSCs and These cells could decrease IL-12 level and reduce the expression of some cell markers of mature DCs. However, the results are contradictory because in the PBMC treated with MSC (Vit E) CM and MSC (Se) CM, IL-10 concentration decreased, IL-12 level and Th1/Treg ratio increased. According to previous studies, the MSC treated with these two antioxidants, might have caused changes in cytokine concentration and gene expression in the PBMC, probably due to its effect on monocytes. Considering the fact that several factors influence the effect of MSC on cells and not all of them studied in this study, a better understanding of the effect of Vit E and Se on MSC requires more studies.
Acknowledgments
We would like to acknowledge Stem Cell Technology Research Center for their supports.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at http://dx.doi.org/10.21037/sci-2020-008
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/sci-2020-008). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the study in ensuring that questions related to the accuracy or reliability of any part of the investigation are appropriately considered and resolved. This study was approved by the ethics board of Iran University of Medical Sciences (IR.IUMS.FMD.REC 1396.9411127004) and informed consent was taken from all individual participants. Furthermore, this work was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Almalki SG, Agrawal DK. Key transcription factors in the differentiation of mesenchymal stem cells. Differentiation 2016;92:41-51. [Crossref] [PubMed]
- Zhao Q, Ren H, Han Z. Mesenchymal stem cells: immunomodulatory capability and clinical potential in immune diseases. Journal of Cellular Immunotherapy 2016;2:3-20. [Crossref]
- Cardenas E, Ghosh R. Vitamin E: a dark horse at the crossroad of cancer management. Biochem Pharmacol 2013;86:845-52. [Crossref] [PubMed]
- Rizvi S, Raza ST, Ahmed F, et al. The role of vitamin e in human health and some diseases. Sultan Qaboos Univ Med J 2014;14:e157-65. [PubMed]
- Hoffmann PR, Jourdan-Le Saux C, Hoffmann FW, et al. A role for dietary selenium and selenoproteins in allergic airway inflammation. J Immunol 2007;179:3258-67. [Crossref] [PubMed]
- Huang Z, Rose AH, Hoffmann PR. The role of selenium in inflammation and immunity: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal 2012;16:705-43. [Crossref] [PubMed]
- Hoffmann PR, Berry MJ. The influence of selenium on immune responses. Mol Nutr Food Res 2008;52:1273-80. [Crossref] [PubMed]
- Stockwin LH, McGonagle D, Martin IG, et al. Dendritic cells: immunological sentinels with a central role in health and disease. Immunol Cell Biol 2000;78:91-102. [Crossref] [PubMed]
- Zhu M, Heydarkhan-Hagvall S, Hedrick M, et al. Manual isolation of adipose-derived stem cells from human lipoaspirates. J Vis Exp 2013;e50585 [Crossref] [PubMed]
- Ge X, Leow SC, Sathiakumar D, et al. Isolation and culture of human adipose-derived stem cells from subcutaneous and visceral white adipose tissue compartments. Bio Protoc 2016;6:e2027 [Crossref]
- Delirezh N, Shojaeefar E, Parvin P, et al. Comparison the effects of two monocyte isolation methods, plastic adherence and magnetic activated cell sorting methods, on phagocytic activity of generated dendritic cells. Cell J 2013;15:218-23. [PubMed]
- Bie Y, Xu Q, Zhang Z. Isolation of dendritic cells from umbilical cord blood using magnetic activated cell sorting or adherence. Oncol Lett 2015;10:67-70. [Crossref] [PubMed]
- El-Sahrigy SA, Mohamed NA, Talkhan HA, et al. Comparison between magnetic activated cell sorted monocytes and monocyte adherence techniques for in vitro generation of immature dendritic cells: an Egyptian trial. Cent Eur J Immunol 2015;40:18-24. [Crossref] [PubMed]
- Zhou H, Li D, Shi C, et al. Effects of Exendin-4 on bone marrow mesenchymal stem cell proliferation, migration and apoptosis in vitro. Scientific Reports 2015;5:12898. [Crossref] [PubMed]
- Spaggiari GM, Abdelrazik H, Becchetti F, et al. MSCs inhibit monocyte-derived DC maturation and function by selectively interfering with the generation of immature DCs: central role of MSC-derived prostaglandin E2. Blood 2009;113:6576-83. [Crossref] [PubMed]
- Zhang Y, Ge XH, Guo XJ, et al. Bone Marrow Mesenchymal Stem Cells Inhibit the Function of Dendritic Cells by Secreting Galectin-1. Biomed Res Int 2017;2017:3248605 [Crossref] [PubMed]
- van den Berk LC, Roelofs H, Huijs T, et al. Cord blood mesenchymal stem cells propel human dendritic cells to an intermediate maturation state and boost interleukin-12 production by mature dendritic cells. Immunology 2009;128:564-72. [Crossref] [PubMed]
- Jung S, Panchalingam KM, Rosenberg L, et al. Ex vivo expansion of human mesenchymal stem cells in defined serum-free media. Stem Cells Int 2012;2012:123030 [Crossref] [PubMed]
- Rescigno M, Martino M, Sutherland CL, et al. Dendritic cell survival and maturation are regulated by different signaling pathways. J Exp Med 1998;188:2175-80. [Crossref] [PubMed]
- van Buul GM, Villafuertes E, Bos PK, et al. Mesenchymal stem cells secrete factors that inhibit inflammatory processes in short-term osteoarthritic synovium and cartilage explant culture. Osteoarthritis Cartilage 2012;20:1186-96. [Crossref] [PubMed]
- Tang J, Cui W, Song F, et al. Effects of mesenchymal stem cells on interleukin-1β-treated chondrocytes and cartilage in a rat osteoarthritic model. Mol Med Rep 2015;12:1753-60. [Crossref] [PubMed]
- Kim JH, Lee YT, Hong JM, et al. Suppression of in vitro murine T cell proliferation by human adipose tissue-derived mesenchymal stem cells is dependent mainly on cyclooxygenase-2 expression. Anat Cell Biol 2013;46:262-71. [Crossref] [PubMed]
- Hernández J, Soto-Canevett E, Pinelli-Saavedra A, et al. In vitro effect of vitamin E on lectin-stimulated porcine peripheral blood mononuclear cells. Vet Immunol Immunopathol 2009;131:9-16. [Crossref] [PubMed]
- Wassall HJ, Devereux G, Seaton A, et al. Complex effects of vitamin E and vitamin C supplementation on in vitro neonatal mononuclear cell responses to allergens. Nutrients 2013;5:3337-51. [Crossref] [PubMed]
- Daeian N, Radfar M, Jahangard-Rafsanjani Z, et al. Selenium supplementation in patients undergoing hematopoietic stem cell transplantation: effects on pro-inflammatory cytokines levels. Daru 2014;22:51. [Crossref] [PubMed]
- Kay AG, Long G, Tyler G, et al. Mesenchymal stem cell-conditioned medium reduces disease severity and immune responses in inflammatory arthritis. Sci Rep 2017;7:18019. [Crossref] [PubMed]
- Ivanova-Todorova E, Bochev I, Dimitrov R, et al. Conditioned medium from adipose tissue-derived mesenchymal stem cells induces CD4+FOXP3+ cells and increases IL-10 secretion. J Biomed Biotechnol 2012;2012:295167 [Crossref] [PubMed]
- Luz-Crawford P, Noel D, Fernandez X, et al. Mesenchymal stem cells repress Th17 molecular program through the PD-1 pathway. PLoS One 2012;7:e45272 [Crossref] [PubMed]
- Rozenberg A, Rezk A, Boivin MN, et al. Human mesenchymal stem cells impact Th17 and Th1 responses through a prostaglandin E2 and myeloid-dependent mechanism. Stem Cells Transl Med 2016;5:1506-14. [Crossref] [PubMed]
- Adolfsson O, Huber BT, Meydani SN. Vitamin E-enhanced IL-2 production in old mice: naive but not memory T cells show increased cell division cycling and IL-2-producing capacity. J Immunol 2001;167:3809-17. [Crossref] [PubMed]
- Zingg JM, Han SN, Pang E, et al. In vivo regulation of gene transcription by alpha- and gamma-tocopherol in murine T lymphocytes. Arch Biochem Biophys 2013;538:111-9. [Crossref] [PubMed]
- Sang LX, Chang B, Zhu JF, et al. Sodium selenite ameliorates dextran sulfate sodium-induced chronic colitis in mice by decreasing Th1, Th17, and gammadeltaT and increasing CD4(+)CD25(+) regulatory T-cell responses. World J Gastroenterol 2017;23:3850-63. [Crossref] [PubMed]
- Broome CS, McArdle F, Kyle JA, et al. An increase in selenium intake improves immune function and poliovirus handling in adults with marginal selenium status. Am J Clin Nutr 2004;80:154-62. [Crossref] [PubMed]
Cite this article as: Ghasemi F, Khoshmirsafa M, Safari E, Asgari M, Alemrajabi M, Nojehdehi S, Khorrami S. Vitamin E and selenium improve mesenchymal stem cell conditioned media immunomodulatory effects. Stem Cell Investig 2021;8:9.


