Effect of mesenchymal stem cells versus aloe vera on healing of deep second-degree burn
Introduction
Skin is the largest organ in the body covering the whole external surface of it and forming about 8% of the total body bulk (1). Skin loss can occur for many reasons; one of the most common reasons is thermal trauma. Worldwide, millions of patients present with skin burns which along with massive cost of treatment, cause mortality and extensive morbidity (2). Burn injuries are variable in terms of the affected tissue, burn depth and degree and complications (3). Burn is classified depending on the lesion severity into first, second and third degree (4).
In deep second-degree burns, patients lack enough skin to cover their burns and the currently used cutaneous substitutes and epithelial grafts are still neither efficient nor effective resolutions. Transplanted skin from donors is currently not a choice due to rejection (5).
So, there was an urgent need to find new modalities to treat deep burns. Aloe vera was one of those new lines of management that has been known as a valuable plant for burns treatment. Extensive studies have described its anti-inflammatory and wound healing effect. It prevents further skin damage and casting of dead epidermis, it increases collagen synthesis, remodeling and enhances wound tensile stress (6). In addition, it shows no rashes, swelling, inflammation, redness, itching or any other toxicity symptoms (7).
Another new modality, not only in the field of treating deep burn but also in the field of regenerative medicine, is mesenchymal stem cells (MSCs). There has been an explosion of literature in the past 10 years to exploit their potential as a source of reparative cells for clinical use in a variety of contexts (8).
MSCs are involved in all three phases of healing to varying degrees. MSCs directly attenuate inflammatory response (9). MSCs can secrete many known mediators of tissue repair, these factors (including growth factors, cytokines and chemokines), stimulate proliferation and migration of the predominant cell types in the wound such as macrophages, endothelial cells, epidermal keratinocytes, and fibroblasts (10). In addition, MSCs provides anti-scarring properties (11).
This study was performed to compare the effect of intradermal injection of bone marrow-mesenchymal stem cells (BM-MSCs) versus topical aloe vera on the healing process of deep second-degree burn after 14 and 21 days in albino rats taking into consideration the unification of the different factors, reported in the previous studies, which may have an effect on healing time.
Methods
Experimental design
Sixty female Wistar albino rats aged 6–7 months and weighing between 200 and 250 g and 10 male albino rats, 6–7 weeks old, weighing around 150 g were used in this study. Animals were acclimatized to their place for one week before the start of the experiment and housed in their cages (one animal/cage). After the approval of the Institutional Research Ethics Committee of Faculty of Medicine, Suez Canal University, female rats were divided randomly into six groups (10 animals/group): Group I: (Control negative): animals were exposed to hair depilation only, Group II: (Aloe vera group): animals were subjected to hair depilation followed by aloe vera gel application, Group III: (MSCs group): animals were subjected to hair depilation followed by intradermal injection of MSCs. Group IV: (Burn group): animals were subjected to hair depilation and thermal induced burn injury, Group V: (Burn and aloe vera group) animals were subjected to hair depilation, burn injury followed by aloe vera gel application and Group VI: (Burn and MSCs group) animals were subjected to hair depilation, burn injury followed by intradermal injection of BM-MSCs.
The study was approved by the Institutional Research Ethics Committee Guidelines (Institutional registration number: IORG0005275), Faculty of Medicine, Suez Canal University, in compliance with National Research Center for Experimental Animals guidelines for the care and use of animals.
Burn induction
Hair on the proximal dorsal region of the back (1.5×1.5 cm2) was removed with commercial depilatory cream. On the next day, animals were subjected to general anesthesia with intramuscular injection of Thiopental Sodium (40 mg/kg) (12). The burn was induced with an iron bar approximately 1.5 cm in diameter and about 50 g in weight. The bar was previously heated in boiling water and it was maintained in contact with the animal skin for 15 s. The pressure exerted on the animal skin corresponded to the weight of the iron bar (4) (Figure 1).
Isolation and culture of MSCs from rat bone marrow
Under sterile conditions, bone marrow from the male rats’ femurs and tibias was flushed with Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% Fetal Calf Serum and 1% Penicillin/Streptomycin (Lonza Bioproducts, Belgium) from one end of the bone and received in a falcon tube from the other end (13). The marrow was washed with phosphate buffer saline (PBS) (Lonza Bioproducts, Belgium) for 5 minutes at 2,000 rpm. Isolated cells were cultured to adjust a density of 1×106 cells per well in full culture media. Cultures were kept at 37 °C in a humidified incubator with 5% CO2 and 95% air infusion. When cells reached ~90% confluence, they were trypsinized with 0.25% trypsin containing 0.02% ethylene diamine tetra acetate (EDTA) (Lonza Bioproducts, Belgium) (14).
Flow cytometry
To assess BM-MSCs immunophenotypically, surface markers (CD 90 and CD 105) were measured in the third passage cultured cells. Specific IOTest conjugated antibodies from eBioscience were added to the test tubes. The samples were mixed, and the tubes were incubated for 20–25 minutes at 18–25 °C in the dark. At room temperature, the tubes were centrifuged for 5 minutes at 150 × g. The pellets were resuspended in 1 ml of PBS in 2–6 °C in a dark room for up to 20 minutes. After centrifugation, pellets were resuspended using 5 ml of PBS and were passed through a 70 mm filter and analyzed for surface marker profile using flow cytometry (CyFlow space–Partec).
Polymerase chain reaction (PCR)
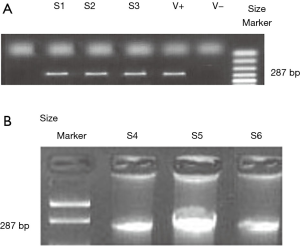
PCR was used for detecting rat male Sry gene sequence in the skin of the female rats. Small pieces of skin from group VI rats were excised. In addition, pieces of skin from normal female and male rats were isolated to be used as negative and positive controls, respectively. PCR amplification of a part of the Sry male gene with a size of 287 bp using primer set with the following sequence: F5’ primer, 5"- GGC TCA ACA GAA TCC CAG CA-3" and R3’ primer 5"- TAG CCC AGT CCT GTC CGT AT -3" (Metabion, Germany).
PCR condition: Using the thermal cycler (Veriti 96 Well, thermal cycler), the following parameters were used: denaturation at 95 °C for 2 minutes followed by 45 cycles of denaturation at 94 °C for 1 minute, annealing at 56 °C for 1 minute, and extension at 72 °C for 2 minutes. For analysis of PCR products, loading buffer was added to each PCR products then they were loaded to the prepared gel and the current was on for 50 minutes.
MSCs administration
Each wound received 1 million cells of BM-MSCs in 500 µL of PBS injected intradermal around the wound at four injection sites.
Aloe Vera gel administration
Aloe vera gel, 1/2 cm2 (Harraz plant medical group, Cairo, Egypt) was applied twice/day topically and left air exposed.
Gross evaluation
Burn wounds were photographed at day 0, 14 and 21 using the same instrument and settings. The following were assessed:
Burn size: The percentage of reduction in burn surface area was calculated as follow: (Area of original wound − area of actual wound)/area of original wound ×100 (15).
Time of complete healing: was recorded as the day on which hairy skin covered the entire burned wound (16).
Microscopic evaluation
Five animals of each group were sacrificed at day 14 and the other five animals were sacrificed at day 21 of the experiment by decapitation under light ether anesthesia. Skin samples from each animal including the wound and 4 mm of the surrounding skin were excised. The specimens were fixed in formalin for 24 hours and then processed to prepare 5 µm thick paraffin sections. The paraffin sections were stained with Hematoxylin and Eosin (H&E) and Masson’s trichrome stain.
Morphometric measurements
Three sections/animal and five fields/section were examined using computer-assisted image analysis. The epidermal thickness in H&E stained section and the optical density and color area percentage of collagen fibers in the Masson’s trichrome stained sections were assessed using an objective lens of magnification ×400 (15). All measurements were detected in randomly chosen non overlapping fields for each section.
Statistical methods
Data obtained using image analyzer was statistically analyzed using the Statistical Package for Social Sciences (SPSS 16 software). The mean and standard deviation for each measurement in each group were calculated. A one-way analysis of variance (ANOVA) test was used followed by post-hoc test to compare the quantitative data of different groups with the control group and burn group, P<0.05 was considered statistically significant.
Results
Characterization of BM-MSCs
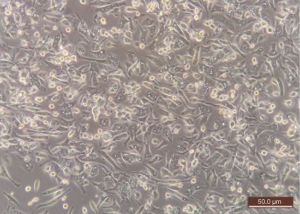
- Morphological assessment: adherent cells were fusiform and elongated, with central nuclei and cytoplasmic prolongations. They reached confluence within 11–14 days (Figure 2).
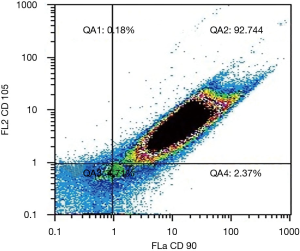
- Immunophenotypic assessment by flow cytometry: third passage cultured cells were 92.74% double positive for CD 90 and CD 105 (Figure 3).
PCR results
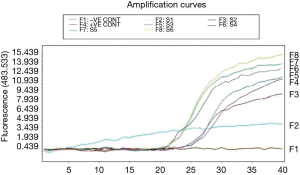
All tested skin samples were positive for the presence of Sry gene (Figures 4,5).
Gross evaluation
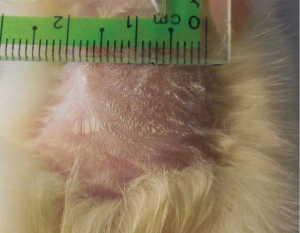
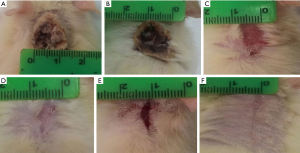
Post burn at day 0, in all burned groups, the skin appeared grayish-white with smooth edges and a diameter that exceeded 1.5 cm and an area of 2.805 cm2 (Figure 6). In burn group, after 14 days, the burned area was covered with dark dry crust (Figure 7A). However, after 21 days, the burned area decreased slowly in all rats (Table 1). Complete healing could not be detected in any rat till the end of the experiment (Figure 7B). In burn and aloe vera group, after 14 days, complete wound healing was detected in all rats (Table 1) with partial hair regaining (Figure 7C), however the complete hairy skin regaining was noticed on day 21 (Figure 7D). In burn and MSCs group, after 14 days, the burned area decreased in all rats with a relatively rapid rate compared to burn group but slower than aloe vera group (Figure 7E and Table 1), however complete regaining of hairy skin in all animals was seen by day 21 (Figure 7F).
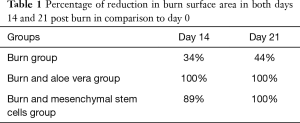
Full table
Histological evaluation
H&E stained sections
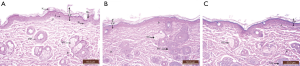
Stained sections of both negative and positive control groups showed normal epidermis, dermis, and hypodermis. The epidermis was thin with 3 to 4 ill-defined strata. Stratum basale consisted of cubical cells with indistinct outline and clear oval nuclei, stratum spinosum is represented by polygonal cells with clear rounded nuclei, stratum granulosum consisted of flattened polygonal granular cells and the outer most layer was the stratum corneum that consists of flattened non-nucleated cells. The dermis showed the papillary and reticular layers. Hair follicles and sebaceous glands were seen in dermis (Figure 8).
Burn group revealed entirely shed epidermis. On day 14, dull, homogeneous eosinophilic tissue masses (coagulative necrosis) surrounded by inflammatory cellular infiltration and numerous dilated congested blood vessels affected the whole skin thickness reaching the muscle layer were detected. These were associated with loss of hair follicles and sebaceous glands (Figure 9A). On day 21, no evidence of epithelialization or skin appendages regeneration was seen, and the necrosis was still there. Also, thin-walled congested blood vessels, focal papillary hemorrhage, inflammatory cellular infiltrate, and few small blood vessels seemed to be newly formed in papillary and reticular layer of dermis were seen (Figure 9B).
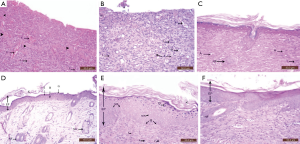
Examination of skin samples from burn and aloe vera group after 14 days revealed, completely regenerated epidermis, which appeared to be thicker than that in the control group. There were no areas of necrosis, however, there were numerous small thin-walled blood vessels seemed to be newly formed and inflammatory cellular infiltration in the dermis. Regenerated hair follicles reappeared in the dermis (Figure 9C). After 21 days, the architecture became nearly similar to the control group with a normal epidermal layer including all its strata. Hair follicles and sebaceous glands reappeared in the reticular layer of the dermis (Figure 9D).
Examination of skin samples in burn and MSCs group after 14 days revealed actively regenerating epidermis with some mitotic activities in the cells of stratum spinosum. The epidermis increased in its thickness in comparison to control and to burn and aloe vera groups with hyperplasia of its basal layer, hyperplasia and hypertrophy of its granular layer and reappearance of the horny layer. Reticular layer of the dermis showed congested blood vessels, inflammatory cellular infiltrate, and many small thin-walled blood vessels less than that seen in the burn group but more than that in the burn and aloe vera group (Figure 9E). After 21 days, the skin appeared nearly like control group but with thicker epidermis. All layers of the epidermis with ill-defined strata were there. Regenerated hair follicles were seen in the reticular layer of the dermis (Figure 9F).
Masson’s trichrome stained sections
Stained sections of both negative and positive control groups showed fine collagen fibers arrangement in the papillary layer that became more abundant and combined into thick coarse irregular interwoven fibers in the reticular layer of the dermis and around the hair follicles (Figure 10).
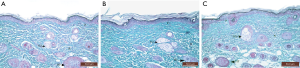
In burn group, the dermis showed disorganized collagen fibers arrangement in the papillary and reticular layers of the dermis after 14 and 21 days (Figure 11A,B).
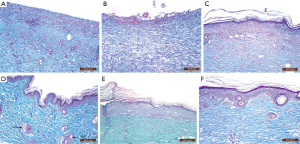
Skin samples from burn and aloe vera group after 14 days showed nearly normal fine collagen fibers arrangement in the dermis. Collagen fibers were less dense than that of control group but denser than that of the burn group (Figure 11C). After 21 days, regaining of normal collagen fibers arrangement in the dermis was seen. Dense collagen fibers were evidenced in the papillary layer and coarse irregular interwoven fibers in the reticular dermis around hair follicles and sebaceous glands (Figure 11D).
Skin samples from burn and MSCs group after 14 days showed disorganized compacted collagen fibers in the dermis which were less dense than that of control group but denser than that of the burn group (Figure 11E). After 21 days, the dermis showed nearly normal dense irregular interwoven collagen fibers arrangement, mainly around the regenerating hair follicles (Figure 11F).
Data analysis
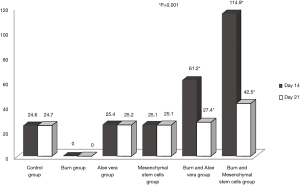
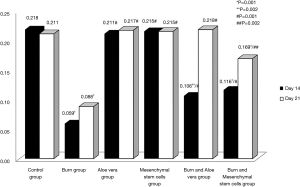
- Epidermal thickness in different experimental groups at days 14 & 21 in H&E stained sections (Table 2, Figure 12): There were no significant differences in the epidermal thickness between both aloe vera group and MSCs group in comparison to control group (P>0.05) at days 14 & 21. There was a statistically significant decrease in epidermal thickness in burn group in comparison to control group (P<0.05) at days 14 & 21. However, there was a statistically significant increase in the epidermal thickness in both burn & aloe vera group and burn & MSCs group (P<0.05) at days 14 & 21 in comparison to control group.
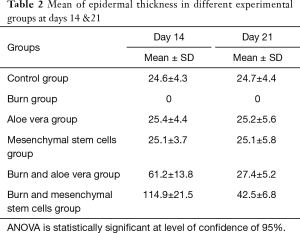 Table 2 Mean of epidermal thickness in different experimental groups at days 14 &21
Table 2 Mean of epidermal thickness in different experimental groups at days 14 &21
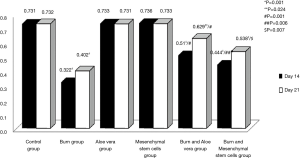
Full table - Optical density of collagen fibers in different experimental groups at days 14 & 21 in Masson’s trichrome stained sections (Table 3, Figure 13): There were no significant differences in the optical density of collagen fibers between both aloe vera group and MSCs group in comparison to control group (P>0.05) at days 14 & 21. There was a statistically significant decrease in optical density of collagen fibers in both burn group and burn & MSCs group in comparison to control group (P<0.05) at days 14 & 21. However, there was a statistically significant decrease in the optical density of collagen fibers in burn & aloe vera group (P<0.05) at day 14 but there was no significant difference (P>0.05) at day 21 in comparison to control group. There was a statistically significant decrease in the optical density of collagen fibers in burn group in comparison to all other experimental groups (P<0.05) at days 14 & 21.
 Table 3 Mean of optical density and color area percentage of collagen fibers in different experimental groups at days 14 &21
Table 3 Mean of optical density and color area percentage of collagen fibers in different experimental groups at days 14 &21
Full table - Color area percentage of collagen fibers in different experimental groups at days 14 & 21 in Masson’s trichrome stained sections (Table 3, Figure 14): There were no significant differences in the color area percentage of collagen fibers between both aloe vera group and MSCs group in comparison to control group (P>0.05) at days 14 & 21. There was a statistically significant decrease in color area percentage of collagen fibers in burn group, burn & aloe vera group and burn & MSCs group in comparison to control group (P<0.05) at days 14 & 21. There was a statistically significant decrease in the color area percentage of collagen fibers in burn group in comparison to all other experimental groups (P<0.05) at days 14 & 21.
Discussion
Burn injuries constitute a major worldwide public health problem and cause more severe physiological stress than other traumas (17).
A wide variety of natural substances have been reported to be useful in the treatment of burn wound. Healing of burn is still a challenge in modern medicine and there are a few drugs capable of accelerating burn healing (18).
The current work revealed severe histopathological changes in burn group, mainly loss of epithelium with its underlying basement membrane. The dermis showed coagulative necrosis surrounded by inflammatory cellular infiltration, numerous dilated congested blood vessels, areas of hemorrhage and few small thin walled blood vessels seemed to be newly formed. Failure of hair regeneration was detected in all animals till the end of the experiment. These findings were documented in many studies such as that done by Tavares Pereira Ddos et al. (4) who found moderate coagulative necrosis at day 14 in deep second degree burned rat. Moreover, Abdel Hamid and Soliman (19) found coagulative necrosis with excessive cellular infiltrate at day 4 in a full thickness burn rat model. Also, Ashkani-Esfahani et al. (20) demonstrated intense inflammatory cellular infiltrate, few congested vessels and failure of epidermal regeneration at day 28 in a second degree burned rats.
The possible explanation of the findings, observed in this group, is that burn injury led to protein denaturation which is represented by coagulative necrosis. Also, the damage applied to cells’ membrane, from the thermal injury, produced a dynamic cascade of inflammatory cellular infiltrate. This is accompanied by increased capillary leakage and rapid proliferation of fibroblasts and this together with the increase in the newly formed blood vessels and the cellular infiltration, generate a mature granulation tissue (21).
In Masson’s trichrome stained sections, in the burn group, the newly deposited collagen was relatively thin and was arranged in different directions in a network like manner. These results are consistent with those of Tavares Pereira Ddos et al. (4) who found loose and irregular collagen fibers deposition in day 14 but denser and irregular collagen deposition at day 21 in deep second degree burned rats. This can be attributed to the extensive thermal injuries that disrupt the balance between collagen synthesis and degradation and leads to abnormalities in collagen metabolism (22).
In aloe vera, treated group, aloe vera showed to be very effective in healing of burn injury; these effects were reflected in the decreased size of wound in gross examination and in the improvement of the histopathological changes. After 14 days, in all animals, gross examination showed complete closure of the burn injury and covering of burned area with regenerated hairy skin. Moreover, the H&E stained sections of this group showed accelerated healing with increased re-epithelialization rates. The dermis showed reappearance of hair follicles, few inflammatory cellular infiltrates and numerous small thin walled blood vessels seemed to be newly formed. On the other hand, after 21 days, the architecture became similar to control group with reappearance of many hair follicles and sebaceous glands.
Our findings, for aloe vera and burn group, are similar to the results obtained by Akhoondinasab, Akhoondinasab, and Saberi (23) who found that aloe vera significantly increase re-epithelialization and shorten the burn wound healing time (at approximately 8 days) in a second degree burn induced with a hot plate in a rat model. Also a study done by Hosseinimehr et al. (24) showed that 0.5% aloe gel enhanced fast re-epithelialization in thermal burn wound induced by hot water at day 25 in a rat model. Furthermore, a randomized control study done by Khorasani et al. (25) showed complete healing in less than 16 days in a second degree burned patients. The difference in healing time between our study and those studies is most probably due to the different methods used for burn induction, different burn extent and different subjects used.
The possible explanation for early wound healing by aloe leaf gel is that it provides several pharmacologically active healing ingredients. Aloe vera glycoprotein contributes to increase epidermal cells activities and the newly formed vessels (19). Also, aloe vera increases epithelial cell migration, blood flow to burn area, decreases inflammatory response and decreases rate of infection (26). In addition, aloe gel attracts water from the dermis and helps to keep this water bound in the stratum corneum producing faster action of its chemical substances. Also, the gel itself forms glue-like substance on skin which acts as a natural “band aide”, sealing in the nutrients (including; gel glycoprotein, mannose-6-phosphate and gibberellin) and allowing them to work immediately (27).
Our results in the Masson’s trichrome stained sections in the aloe vera treated animals showed fine collagen fibers arrangement in the dermis in day 14, and normal collagen fibers arrangement after 21 days. These results are consistent with Abdel Hamid and Soliman (19) who suggested that treatment with aloe progressively increased collagen content in the granulation tissue by day 12 in full-thickness skin burned rats. Other studies in a wound model by MacKay and Miller and Tarameshloo et al. (28,29) suggested that treatment with aloe vera increases stimulation of fibroblast and in turn the collagen content.
Subramanian et al. (30) attributed the increase in collagen synthesis to the gel’s mannose-6-phosphate. Mannose- containing products have been shown to increase macrophage activity and therefore stimulate fibroblast activity and collagen synthesis (31). On the other hand, other researchers claimed that gibberellin, a growth hormone, interacts with receptor on the fibroblasts, thereby stimulating its activity and proliferation, which in turn accelerated collagen synthesis after application of aloe gel (32).
Another explanation for the healing effect of aloe gel lies in how it acts on cell proliferation of the dermal component. Plant’s glycoprotein stimulates both the fibroblasts and the keratinocytes to produce fibronectin (25). Also aloe gel has a unique ability to increase production of fibroblast cells between six and eight times faster than normal cell production (33). In addition, it not only increases collagen content of the wound but also increases the degree of collagen cross linking (34).
Also, Tabandeh et al. (35) postulated that aloe gel regulate expression of matrix metalloproteinase-3 gene (MMP-3 gene) and tissue inhibitors of matrix metalloproteinase-2 gene (TIMP-2 gene), an endogenous inhibitor to MMP-3. The activity of MMP-3 is particularly important in regulating the final stage healing progression. MMP-3 expression may influence the granulation tissue formation and wound closure by increase production of glycosaminoglycans and collagen.
The other modality used for burn healing is BM-MSCs. The burn injury in our experimental rats demonstrated complete gross healing by the end of the 21st day. However, H&E stained sections, after 14 days, showed complete epithelialization of the burn surface with prominent mitotic figures. The dermis showed wider and more cellular inflammatory tissue, many small thin walled blood vessels seemed to be newly formed and few regenerated hair follicles compared to burn group. In contrast, after 21 days, BM-MSCs treated group revealed architecture nearly similar to control group but with thicker epidermis.
The previous finding was further supported by Liu et al. (36) who demonstrated burn injury epithelialization at day 11 after human umbilical cord MSCs transplantation in 30% total body surface area with a full-thickness burned rats. Moreover, in consistence with our results, Huang et al. and Wan et al. (37,38) noticed that MSCs has the ability to promote the formation of new skin appendages after wound injury. These findings can be explained by MSCs migration to the injured sites and localization of them in the epidermis and hair follicles of the burned skin (36). Good efficacy of MSCs might be related to soluble growth factors production (epidermal growth factor, vascular endothelial growth factor, keratinocyte growth factor and fibroblast growth factor) that influences their local environment by activation of resident cells (39). Also, Khosrotehrani and Kim and Cho (40,41) studies reported that native skin MSCs populate the normal skin niche, remain quiescent and become active after injury, aiding in healing through their paracrine signaling. Interestingly, previous studies done by Sasaki et al., Griffin et al., Yew et al. and J. Kim et al. (42-45) reported that MSCs promote burn healing by differentiating into different cell types within the injury and increasing the rate of re-epithelialization and increasing dermal matrix production.
Our results showed that treatment with BM-MSCs significantly increased collagen deposition compared to burn group as Masson’s trichrome stained sections, showed dense disorganized collagen deposition at day 14 and denser irregular interwoven collagen fibers deposition in the dermis at day 21. This was confirmed by the statistically significant increase of the optical density and color area percentage of collagen fibers of this group compared to the burn group. This result is in accordance with the results of Clover et al. (46) who showed significant increase in the collagen density at day 14 compared to burn group in a second degree burned pig. Also, in a rat wound model, Basiouny et al. (14) found that after three weeks of treatment with topical MSCs, collagen fibers deposition became thicker and were arranged in different directions.
It was stated that the primary trophic property of MSCs to improve tissue repair is the paracrine interaction with resident cells, formation of extracellular matrix or their differentiation into resident cells (47). MSCs secrete growth factors that regulate the dermal fibroblast, proliferation and migration and angiogenesis (48,49). They attributed their findings to the initial deposition of thin collagen fibrils (type III) that were later resorbed and replaced with thicker fibrils (type I) indicating maturity of burn wound (50).
Indeed, the present study has pointed out so far that MSCs in general are safe. No serious adverse reaction reported in the BM-MSCs group most probably due to anti-inflammatory effect and immunomodulatory properties of MSCs (51-53).
Effect of aloe vera was found to be much better than BM-MSCs in accelerating burn wound healing. This may be due to the loss of some stem cells or their failure to home to the burned skin. Optimization of process of treatment with MSCs may improve burn healing results.
Acknowledgments
The authors thank Associate Professor Horeya Erfan Korayem and Dr. Hend Marof for their help through this work.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/sci-2020-030). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the Institutional Research Ethics Committee Guidelines (Institutional registration number: IORG0005275), Faculty of Medicine, Suez Canal University, in compliance with National Research Center for Experimental Animals guidelines for the care and use of animals.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Riccobono D, François S, Valente M, et al. Advances in Stem Cell Therapy: Specific Applications in the Treatment of Cutaneous Radiation Syndrome. J Stem Cell Res Ther 2014;4:2. [Crossref]
- Khodja AN, Mahlous M, Tahtat D, et al. Evaluation of healing activity of PVA/chitosan hydrogels on deep second degree burn: pharmacological and toxicological tests. Burns 2013;39:98-104. [Crossref] [PubMed]
- Evers LH, Bhavsar D, Mailänder P. The biology of burn injury. Exp Dermatol 2010;19:777-83. [Crossref] [PubMed]
- Tavares Pereira Ddos S, Lima-Ribeiro MH, de Pontes-Filho NT, et al. Development of animal model for studying deep second-degree thermal burns. J Biomed Biotechnol 2012;2012:460841 [Crossref] [PubMed]
- Arno A, Smith AH, Blit PH, et al. Stem cell therapy: A new treatment for burns? Pharmaceuticals 2011;4:1355-80. [Crossref] [PubMed]
- Tarameshloo M, Norouzian M, Zarein-Dolab S, et al. Aloe vera gel and thyroid hormone cream may improve wound healing in Wistar rats. Anat Cell Biol 2012;45:170-7. [Crossref] [PubMed]
- Moghbel A, Hematti A, Ghalambor A, et al. Wound healing and toxicity evaluation of Aloe Vera cream. Toxocology Lett 2007;S233. [Crossref]
- Francis E, Kearney L, Clover J. The effects of stem cells on burn wounds: a review. Int J Burns Trauma 2019;9:1-12. [PubMed]
- Aggarwal S, Pittenger M. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 2005;105:1815-22. [Crossref] [PubMed]
- Chen L, Tredget E, Wu P, et al. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS One 2008;3:e1886 [Crossref] [PubMed]
- Colwell AS, Beanes SR, Soo C, et al. Increased angiogenesis and expression of vascular endothelial growth factor during scarless repair. Plast Reconstr Surg 2005;115:204-12. [PubMed]
- Khorasani G, Hosseinimehr SJ, Zamani P, et al. The Effect of Saffron (Crocus Sativus) Extract for Healing of Second-degree Burn Wounds in Rats. Keio J Med 2008;57:190-5. [Crossref] [PubMed]
- Dickinson H, Milton P, Jenkin G. The isolation and characterization of putative mesenchymal stem cells from the spiny mouse. Cytotechnology 2012;64:591-9. [Crossref] [PubMed]
- Basiouny HS, Salama NM, Mohamed Z, et al. Effect of Bone Marrow Derived Mesenchymal Stem Cells on Healing of Induced Full-Thickness Skin Wounds in Albino Rat. Int J Stem Cells 2013;6:12-25. [Crossref] [PubMed]
- Li Y, Zheng L, Xu X, et al. Mesenchymal stem cells modified with angiopoietin-1 gene promote wound healing. Stem Cell Res Ther 2013;4:113. [Crossref] [PubMed]
- Borena BM, Pawde AM, Amarpal HP, et al. Evaluation of autologous bone marrow-derived nucleated cells for healing of full-thickness skin wounds in rabbits. Int Wound J 2010;7:249-60. [Crossref] [PubMed]
- Xue L, Xu Y, Xie J, et al. Effects of human bone marrow mesenchymal stem cells on burn injury healing in a mouse model. Int J Clin Exp Pathol 2013;6:1327-36. [PubMed]
- Öhnstedt E, Lofton Tomenius H, et al. The discovery and development of topical medicines for wound healing. Expert Opin Drug Discov 2019;14:485-97. [Crossref] [PubMed]
- Abdel Hamid AA, Soliman MF. Effect of topical aloe vera on the process of healing of full-thickness skin burn : a histological and immunohistochemical study. J Histol Histopathol 2015;2:3. [Crossref]
- Ashkani-Esfahani S, Imanieh M, Khoshneviszadeh M, et al. The Healing Effect of Arnebia Euchroma in Second Degree Burn Wounds in Rat as an Animal Model. Iran Red Crescent Med J 2012;14:70-4. [PubMed]
- Busuioc CJ, Mogoşanu GD, Popescu FC, et al. Phases of the cutaneous angiogenesis process in experimental third-degree skin burns: histological and immunohistochemical study. Rom J Morphol Embryol 2013;54:163-71. [PubMed]
- Rea S, Giles NL, Webb S, et al. Bone marrow-derived cells in the healing burn wound — More than just inflammation. Burns 2009;35:356-64. [Crossref] [PubMed]
- Akhoondinasab MR, Akhoondinasab M, Saberi M. Comparison of Healing Effect of Aloe Vera Extract and Silver Sulfadiazine in Burn Injuries in Experimental Rat Model. World J Plast Surg 2014;3:29-34. [PubMed]
- Hosseinimehr SJ, Khorasani G, Azadbakht M, et al. Effect of aloe cream versus silver sulfadiazine for healing burn wounds in rats. Acta Dermatovenerol Croat 2010;18:2-7. [PubMed]
- Khorasani G, Hosseinimehr SJ, Azadbakht M, et al. Aloe versus silver sulfadiazine creams for second-degree burns: A randomized controlled study. Surg Today 2009;39:587-91. [Crossref] [PubMed]
- Bhuvana KB, Hema NG, Patil RT. Review on Aloe Vera. Int J Adv Res 2014;2:677-91.
- Dal'Belo SE, Gaspar LR, Maia Campos PM. Moisturizing effect of cosmetic formulations containing aloe vera extract in different concentrations assessed by skin bioengineering techniques. Skin Res Technol 2006;12:241-6. [Crossref] [PubMed]
- MacKay D, Miller A. Nutritional support for wound healing. Altern Med Rev 2003;8:359-77. [PubMed]
- Tarameshloo M, Norouzian M, Zarein-Dolab S, et al. A comparative study of the effects of topical application of Aloe vera, thyroid hormone and silver sulfadiazine on skin wounds in Wistar rats. Lab Anim Res 2012;28:17-21. [Crossref] [PubMed]
- Subramanian S, Kumar DS, Arulselvan P. Wound healing potential of aloe vera leaf gel studied in experimental rats. Asian J Biochem 2006;1:178-85. [Crossref]
- Khan AW, Kotta S, Ansari SH, et al. Formulation development, optimization and evaluation of aloe vera gel for wound healing. Pharmacogn Mag 2013;9:S6-10. [Crossref] [PubMed]
- Heggers JP, Kucukcelebi A, Listengarten D, et al. Beneficial effect of Aloe on wound healing in an excisional wound model. J Altern Complement Med. 1996;2:271-7. [Crossref] [PubMed]
- Sajjad A, Subhani Sajjad S. Aloe vera: An Ancient Herb for Modern Dentistry — A Literature Review. J Dent Surg 2014. doi:
10.1155/2014/210463 .10.1155/2014/210463 - Shahzad MN, Ahmed N. Effectiveness of Aloe Vera Gel Compared with 1% Silver Sulphadiazine Cream as Burn Wound Dressing in Second Degree Burns. J Pak Med Assoc 2013;63:225-30. [PubMed]
- Tabandeh MR, Oryanb A, Mohammadalipourb A. Polysaccharides of Aloe vera induce MMP-3 and TIMP-2 gene expression during the skin wound repair of rat. Int J Biol Macromol 2014;65:424-30. [Crossref] [PubMed]
- Liu L, Yu Y, Hou Y, et al. Human Umbilical Cord Mesenchymal Stem Cells Transplantation Promotes Cutaneous Wound Healing of Severe Burned Rats. PLoS One 2014;9:e88348 [Crossref] [PubMed]
- Huang S, Lu G, Wu Y, et al. Mesenchymal stem cells delivered in a microsphere-based engineered skin contribute to cutaneous wound healing and sweat gland repair. J Dermatol Sci 2012;66:29-36. [Crossref] [PubMed]
- Wan J, Xia L, Liang W, et al. Transplantation of Bone Marrow-Derived Mesenchymal Stem Cells Promotes Delayed Wound Healing in Diabetic Rats. J Diabetes Res 2013;2013:647107 [Crossref] [PubMed]
- Hong HS, Son Y. Substance-p-mobilized Mesenchymal Stem Cells Accelerate Skin Wound Healing. Tissue Eng Regen Med 2014;11:483-91. [Crossref]
- Khosrotehrani K. Mesenchymal stem cell therapy in skin : why and what for ? Exp Dermatol 2013;22:307-10. [Crossref] [PubMed]
- Kim N, Cho S. Clinical applications of mesenchymal stem cells. Korean J Intern Med 2013;28:387-402. [Crossref] [PubMed]
- Sasaki M, Abe R, Fujita Y, et al. Mesenchymal stem cells are recruited into wounded skin and contribute to wound repair by transdifferentiation into multiple skin cell type. J Immunol. J Immunol 2008;180:2581-7. [Crossref] [PubMed]
- Griffin M, Iqbal SA, Sebastian A, et al. Degenerate wave and capacitive coupling increase human MSC invasion and proliferation while reducing cytotoxicity in an in vitro wound healing model. PLoS One 2011;6:e23404 [Crossref] [PubMed]
- Yew TL, Hung YT, Li HY, et al. Enhancement of wound healing by human multipotent stromal cell conditioned medium: the paracrine factors and p38 MAPK activation. Cell Transplant 2011;20:693-706. [Crossref] [PubMed]
- Kim JW, Lee JH, Lyoo YS, et al. The effects of topical mesenchymal stem cell transplantation in canine experimental cutaneous wounds. Vet Dermatol 2013;24:242-e53. [Crossref] [PubMed]
- Clover AJ, Kumar AH, Isakson M, et al. Allogeneic mesenchymal stem cells, but not culture modified monocytes, improve burn wound healing. Burns 2015;41:548-57. [Crossref] [PubMed]
- Doorn J, Moll G, Blanc K, Le , et al. Therapeutic applications of mesenchymal stromal cells: paracrine effects and potential improvements. Tissue Eng Part B Rev 2012;18:101-15. [Crossref] [PubMed]
- Hocking AM, Gibran NS. Mesenchymal stem cells: paracrine signaling and differentiation during cutaneous wound repair. Exp Cell Res 2010;316:2213-19. [Crossref] [PubMed]
- Smith AN, Willis E, Chan VT, et al. Mesenchymal stem cells induce dermal fibroblast responses to injury. Exp Cell Res 2010;316:48-54. [Crossref] [PubMed]
- Isackson D, Cook KJ, McGill LD, et al. Mesenchymal stem cells increase collagen infiltration and improve wound healing response to porous titanium percutaneous implants. Med Eng Phys 2013;35:743-53. [Crossref] [PubMed]
- Hass R, Kasper C, Böhm S, et al. Different populations and sources of human mesenchymal stem cells (MSC): A comparison of adult and neonatal tissue-derived MSC. Cell Commun Signal 2011;9:12. [Crossref] [PubMed]
- Lalu MM, McIntyre L, Pugliese C, et al. Safety of cell therapy with mesenchymal stromal cells (SafeCell): a systematic review and meta-analysis of clinical trials. PLoS One 2012;7:e47559 [Crossref] [PubMed]
- Steffens D, Leonardi D, Rigon P, et al. Development of a new nanofiber scaffold for use with stem cells in a third degree burn animal model. Burns 2014;40:1650-60. [Crossref] [PubMed]
Cite this article as: Imbarak N, Abdel-Aziz HI, Farghaly LM, Hosny S. Effect of mesenchymal stem cells versus aloe vera on healing of deep second-degree burn. Stem Cell Investig 2021;8:12.