CD34+ therapy-related acute promyelocytic leukemia in a patient previously treated for breast cancer
Introduction
Acute promyelocytic leukemia (APL) comprises 10% of cases of de novo acute myeloid leukemia (AML) and is characterized by a balanced translocation involving the RARA gene, most frequently the t(15;17)(q22;q12) (1). Rarely, APL can occur as a secondary leukemia after therapy for another malignancy (t-APL) (2-4). APL has a distinct morphology, including promyelocytes with prominent azurophilic granules (5), and a distinct immunophenotype (most often CD13+, CD33+, CD117+, CD34−, HLA-DR−) (6) compared with other subtypes of AML. The prognosis for patients with APL is excellent overall, however, many patients die within the first week of therapy due to complications of disseminated intravascular coagulation (1). It is imperative that the diagnosis of APL be made promptly and treatment initiated immediately in order to prevent this complication.
Currently we present the case of a woman who presented with pancytopenia after chemotherapy for breast cancer. The presence of CD34+ blasts misled the referring physicians into considering a diagnosis of therapy related AML (t-AML).
Case presentation
A 61-year-old woman presented in November 2015 with complaints of fatigue and an unresolving sinus infection. A complete blood count (CBC) showed a white blood count (WBC) of 500/mm3, a hemoglobin of 4.6 g/dL and a platelet count of 28,000/mm3. The patient had a prior history of stage IIB, T2N1, estrogen receptor positive, progesterone receptor positive, HER2/neu negative, poorly differentiated invasive ductal carcinoma of the right breast diagnosed in 2011 that was treated with bilateral mastectomy and axillary lymph node dissection. Postoperative chemotherapy consisted of 4 cycles of dose dense Adriamycin and cyclophosphamide followed by 4 cycles of paclitaxel and then radiation therapy. The patient was currently receiving anastrozole. She also had a history of hypertension and paroxysmal atrial fibrillation. The patient had a bone marrow aspiration and biopsy performed by a radiologist at an outside institution. The aspirate smears were hemodilute and markedly hypocellular. Rare hypergranular promyelocytes were noted. On flow cytometry, 4% of the cells were immature myeloid cells with the immunophenotype CD34+, CD117+, CD13+, and CD33+. The core biopsy was markedly hypercellular (>95%) with CD34+ myeloblasts representing 60–70% of the cells present. The patient was transferred to us for therapy.
Upon evaluation at our institution the WBC was 1300/mm3, the hemoglobin (post transfusion) was 8.7 g/dL, and the platelet count was 19,000/mm3. There were 13% neutrophils, 69% lymphocytes, 1% eosinophils, 1% basophils and 16% blasts in the peripheral blood. The prothrombin time was 12.2 s, the activated partial thromboplastin time was 24.6 s and the fibrinogen was 256 mg/dL. D-dimers were present at a level of greater than 35.2 mg/L.
Due to the presence of pancytopenia and an abnormal coagulation profile, all-trans retinoic acid (ATRA) was immediately administered to the patient pending clarification of her diagnosis. A bone marrow aspirate at our institution showed that the smears were cellular with 50% abnormal promyelocytes and 23% myeloblasts (Figure 1). The promyelocytes and blasts were mostly intermediate in size with scant to moderate cytoplasm; some had cytoplasmic granules and blebs and some had bilobed nuclei. Flow cytometry demonstrated an abnormal immature myeloid population (37% of total) that was CD13+, CD33+, CD34+ and CD117+ with aberrant expression of CD2 while negative for HLA-DR, CD11b, CD11c and CD56 (Figure 2). The bone marrow core was hypercellular (90%) and was infiltrated with sheets of immature myeloid precursors and blasts that were large to intermediate in size with moderate cytoplasm, round to slightly irregular nuclear contour and fine chromatin. FISH demonstrated the PML-RARA fusion in 142 of 200 cells. PML-RAR fusions transcripts were detected by RT-PCR at a level of 0.15738. Cytogenetics subsequently demonstrated 46,XX,t(15;17)(q24;q21)[16]/46,idem,del(X)(p11.3),add(3)(q29),t(4;5)(q31;q11.2)[3]/46,XX[1]. No molecular abnormalities were detected on an MDS/AML nextgen sequencing panel.
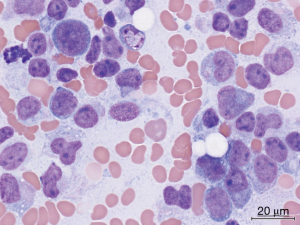
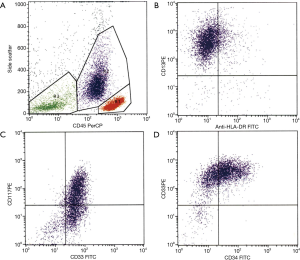
The patient received induction therapy with ATRA, idarubicin 12 mg/m2 daily times 3 and cytarabine 100 mg/m2 IV continuous infusion times 7 days. Her induction was uneventful including no evidence of differentiation syndrome. She obtained complete remission by day 35 of therapy. She is currently receiving a planned course of consolidation therapy with chemotherapy, ATRA and arsenic trioxide.
Discussion
As cancer therapies have improved, more patients are surviving their first cancer and thus are at risk of developing long term complications such as t-AML. Most cases of t-AML can be categorized into one of two broad categories. In the first, patients present 1–3 years after their initial therapy, have no antecedent cytopenias, and typically have cytogenetic abnormalities involving chromosome 11q23 (7). In the other group, patients present 3–5 years after therapy, have a myelodysplastic phase, and frequently have poor risk cytogenetics including −5, −7 and complex abnormalities (8). Breast cancer survivors are at relatively high risk for the development of t-AML, in part due to their excellent prognosis for surviving their breast cancer (9). In an NSAPB report, patients who had received dose intensive Adriamycin and cyclophosphamide plus growth factor had a risk of 1.01% at 5 years (10). The addition of radiation further increased the risk of t-AML (relative risk, 2.38).
Most patients with t-AML have a poor prognosis (11). However patients with good risk cytogenetics can have a prognosis similar to that of de novo patients. In a study of 206 patients with therapy-related myeloid neoplasms (t-MN) reported by Larson the median survival was 7–9 months overall, however it was 27 months in patients with favorable cytogenetics (12). In a GIMEMA study of 51 patients with t-APL (13), the complete remission, 4-year event-free survival, and 4-year overall survival rates were 97% vs. 93%, 65% vs. 68%, and 85% vs. 78% for the t-APL and de novo APL groups, respectively, after therapy with the AIDA regimen. In another study of 106 t-APL patients (60 of who had prior breast cancer) the actuarial survival at 8 years was 59% after either an anthracycline and cytarabine-based regimen or ATRA combined with chemotherapy (14). However, in a more recent report, patients with t-APL had an inferior CR rate (63.6% vs. 92.5%, P=0.008), largely due to a higher induction mortality rate (2). Although there were no cases of leukemic resistance in either group in the latter study, the increased induction mortality translated into an inferior overall survival rate (51% vs. 84%, P<0.005).
Classically, APL cells are CD13+, CD33+, CD117+, CD34−, and HLA-DR−, however this immunophenotype is not present in all cases (5). Gorczyca et al. described four distinct patterns in 97 cases of APL (15). The majority of cases (classical hypergranular APL) were characterized by high side scatter (SSC), CD117+, CD34−, heterogeneous CD13+ and bright CD33+. The second most common type (microgranular) differed from classical APL by low SSC, and frequent coexpression of CD2 and CD34. Rare cases showed a mixture of neoplastic cells (low SSC/CD2+/CD13+/CD34+/CD117+) and a prominent population of benign granulocytes and maturing myeloid precursors. A final subtype showed two APL populations, one with hypogranular and one with hypergranular characteristics. In some reports (16), as many as one third of APL patients are CD34+. These patients most often have hypogranular morphology and a higher WBC at presentation. In a report by Liang et al., the median WBC was 25,900/mm3 for patients with CD34+ APL compared with 5,300/mm3 for patients with CD34− APL (17). Although this author found no difference in the induction CR rate, others have found that CD34 expression was significantly associated with a decreased incidence of molecular remission (16), increased incidence of early death (16), as well as decreased disease free survival (18) and overall survival (18). Of note CD34+ APL cells can be variably responsive to ATRA, suggesting that chemotherapy might still be necessary in these patients (19).
The presence of CD34+ cells and hypogranular morphology can mislead the physician. Mohamed et al. reported a patient with pancytopenia whose bone marrow morphology demonstrated 90% blasts that were mostly agranular with no Auer rods (20). Flow cytometry demonstrated that the cells were CD13+, CD33+, CD117+ and MPO+, as well as CD34+ and HLA-DR+. The patient started standard treatment for AML, but the therapy was changed after cytogenetics demonstrated t(15;17)(q22;q12). Our patient was similar to theirs, however ours was more misleading because the history of prior treatment for breast cancer led the physicians to believe this was a more typical t-AML.
Acknowledgements
None
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this Case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
References
- Fenaux P, Chomienne C, Degos L. Acute promyelocytic leukemia: biology and treatment. Semin Oncol 1997;24:92-102. [PubMed]
- Elliott MA, Letendre L, Tefferi A, et al. Therapy-related acute promyelocytic leukemia: observations relating to APL pathogenesis and therapy. Eur J Haematol 2012;88:237-43. [Crossref] [PubMed]
- Duffield AS, Aoki J, Levis M, et al. Clinical and pathologic features of secondary acute promyelocytic leukemia. Am J Clin Pathol 2012;137:395-402. [Crossref] [PubMed]
- Rashidi A, Fisher SI. Therapy-related acute promyelocytic leukemia: a systematic review. Med Oncol 2013;30:625. [Crossref] [PubMed]
- Bennett JM, Catovsky D, Daniel MT, et al. Proposals for the classification of the acute leukaemias. French-American-British (FAB) co-operative group. Br J Haematol 1976;33:451-8. [Crossref] [PubMed]
- Paietta E, Andersen J, Gallagher R, et al. The immunophenotype of acute promyelocytic leukemia (APL): an ECOG study. Leukemia 1994;8:1108-12. [PubMed]
- Super HJ, McCabe NR, Thirman MJ, et al. Rearrangements of the MLL gene in therapy-related acute myeloid leukemia in patients previously treated with agents targeting DNA-topoisomerase II. Blood 1993;82:3705-11. [PubMed]
- Le Beau MM, Albain KS, Larson RA, et al. Clinical and cytogenetic correlations in 63 patients with therapy-related myelodysplastic syndromes and acute nonlymphocytic leukemia: further evidence for characteristic abnormalities of chromosomes no. 5 and 7. J Clin Oncol 1986;4:325-45. [PubMed]
- Martin MG, Welch JS, Luo J, et al. Therapy related acute myeloid leukemia in breast cancer survivors, a population-based study. Breast Cancer Res Treat 2009;118:593-8. [Crossref] [PubMed]
- Smith RE. Risk for the development of treatment-related acute myelocytic leukemia and myelodysplastic syndrome among patients with breast cancer: review of the literature and the National Surgical Adjuvant Breast and Bowel Project experience. Clin Breast Cancer 2003;4:273-9. [Crossref] [PubMed]
- Churpek JE, Larson RA. The evolving challenge of therapy-related myeloid neoplasms. Best Pract Res Clin Haematol 2013;26:309-17. [Crossref] [PubMed]
- Larson RA, Le Beau MM. Prognosis and therapy when acute promyelocytic leukemia and other "good risk" acute myeloid leukemias occur as a therapy-related myeloid neoplasm. Mediterr J Hematol Infect Dis 2011;3:e2011032. [Crossref] [PubMed]
- Pulsoni A, Pagano L, Lo Coco F, et al. Clinicobiological features and outcome of acute promyelocytic leukemia occurring as a second tumor: the GIMEMA experience. Blood 2002;100:1972-6. [Crossref] [PubMed]
- Beaumont M, Sanz M, Carli PM, et al. Therapy-related acute promyelocytic leukemia. J Clin Oncol 2003;21:2123-37. [Crossref] [PubMed]
- Gorczyca W. Acute promyelocytic leukemia: four distinct patterns by flow cytometry immunophenotyping. Pol J Pathol 2012;63:8-17. [PubMed]
- Ahmad EI, Akl HKh, Hashem ME, et al. The biological characteristics of adult CD34+ acute promyelocytic leukemia. Med Oncol 2012;29:1119-26. [Crossref] [PubMed]
- Liang JY, Wu DP, Liu YJ, et al. Clinical and laboratory features of patients with CD34(+) acute promyelocytic leukemia. Zhonghua Zhong Liu Za Zhi 2009;31:196-8. [PubMed]
- Lee JJ, Cho D, Chung IJ, et al. CD34 expression is associated with poor clinical outcome in patients with acute promyelocytic leukemia. Am J Hematol 2003;73:149-53. [Crossref] [PubMed]
- Foley R, Soamboonsrup P, Carter RF, et al. CD34-positive acute promyelocytic leukemia is associated with leukocytosis, microgranular/hypogranular morphology, expression of CD2 and bcr3 isoform. Am J Hematol 2001;67:34-41. [Crossref] [PubMed]
- Mohamed M, Dun K, Grabek J. Atypical features in a patient with acute promyelocytic leukaemia: a potential diagnostic pitfall. BMJ Case Rep 2013;2013.
Cite this article as: Savooji J, Shakil F, Islam H, Liu D, Seiter K. CD34+ therapy-related acute promyelocytic leukemia in a patient previously treated for breast cancer. Stem Cell Investig 2016;3:7.


